Name________________________ Quarter Final 1—Study Guide
advertisement

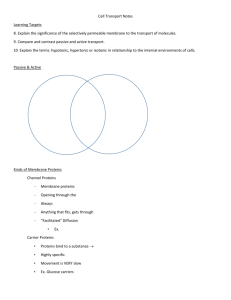
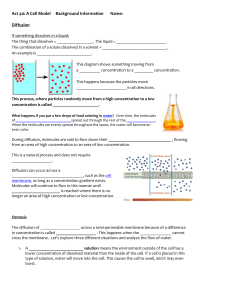
Name________________________ Quarter Final 1—Study Guide Scientific Method Scientific Method: Define the following terms as they have to do with the scientific method. 1. Name the steps of the scientific method. 1. 2. 3. 4. 5. 6. Ask a question Form a hypothesis Experiment Collect data Analyze data Form a conclusion 2. What is the difference between a Theory and a Law? Can a Theory become a Law? Why or why not? 3. What makes a good scientific question? It must be testable. In other words, you must be able to do an experiment to collect data to answer it. Why is the question, “Do roses smell better than daffodils?” not a good question to answer scientifically? It is an opinion question; it is not testable. Characteristics of Life A. List and explain each of the seven (7) characteristics of life in your own words. 1.__Grow and develop______________________ 2.___Reproduce_____________________ 3.__DNA______________________ 4.______Homeostasis__________________ 5.______Evolve__________________ 6.____Adapt____________________ 7.______Made of cells__________________ B. Label the parts of a virus on the picture below. C. What type of virus is the above picture?____Bacteriophage________________________ What organisms do these viruses infect? Bacteria D. What three materials are viruses made of? 1. Lipids 2. Proteins 3. DNA or RNA E. What type of organism is shown in figure 18.2? _____Prokaryote (bacteria)______________ Describe the two parts it possesses that enables it to move. Cilia and Flagella Chemistry of Life A. Know how to find numbers of protons, neutrons, and electrons in any element. B. Know the charges of each of the subatomic particles in part A. C. What type of elements on the periodic table form covalent bonds? nonmetal + nonmetal_____________ D. A bond that is formed when 2 atoms share electrons is a(n) ___covalent_______ bond, while a bond that is formed when one atoms gives an electron to another atom is called a(n) __ionic_______________ bond. E. What is an ion? An atom that has gained or lost an electron Explain how ions are formed. E. What is an isotope? An element with a different number of neutrons F. Draw a water molecule in the space below showing its polar charges. G. What causes the water molecule that you drew to be polar? Explain. Differences in pull of the negative electrons H. What causes water molecules to form H-bonds? The positive ends of the water bond to the negative ends of neighboring water molecules. I. What is an acid? A solution with more H+ What is a base? A solution with more OHA base has more ___OH-______ions, while an acid has more ____H+__________ ions. A pH of a 2 would be very _Acidic____, while a pH of a 13 would be very __basic______________. K. If Tomato Juice has a pH of 4, and Orange Juice has a pH of 3, finish this statement. Orange Juice is _10______times more acidic than Tomato Juice. If Melons have a pH of 6 and cherries have a pH of 4, then Cherries are ___100____ times more acidic than Melons. L. A substance that neutralizes small amounts of acids or bases added to a solution is a _buffer______________. Molecules of Life 1. Why is Carbon the backbone of life? Carbon has 4 valence electrons allowing it bond with itself and many other elements. It is in every organic molecule. 2. List the 4 organic molecules. Carbohydrates, lipids, proteins, nucleic acids 3. What is the monomer of a: carbohydrate? ___monosaccharide___________ Lipid____fatty acid___________________ Protein___amino acid_________________ Nucleic Acid___nucleotide__________________ 4. What atoms are present in a: carb? __CHO 1:2:1 ratio____________ Lipid_______CHO________________ Protein_____CHON_______________ Nucleic Acid____CHONP_________________ 5. What are the different types of carbohydrates and the functions of each? 1. monosaccharide- simple sugars, quick energy 2. disaccharide- simple sugars, quick energy 3. polysaccharide- complex sugars for longer term storage 3 types of polysaccharides: Starch- plant energy storage Glycogen- animal energy storage Cellulose-structure and support in cell walls 6. What are the different types of lipids and the functions of each (include phospholipids)? Phospholipids- in the cell membrane Waxes- waterproof covering Fats-long term animal energy storage Oils-short term energy storage 7. What is the difference between a saturated and unsaturated fat? Where do they each come from? Draw a picture to explain. Saturated come from animals and all of the carbons have a hydrogen attached to them, unsaturated from plants and have at least one double bond 8. What is the function of a protein? Building blocks of cells, catalyze (start) chemical reactions for cells in the form of enzymes -What makes each of the amino acids different? Each has a different R group -How many are there? 20 10 hydrophilic and 10 hydrophobic 9. What is the function of nucleic acids? Contain the genetic information for organisms 10. Label each of the macromolecules as one of the 4 organic molecules. Be able to recognize these! A=carbs B=proteins C=nucleic acids D=lipids Cell Organelles 1. List the three parts of the cell theory. Cells are the basic unit of life. All organisms are made of cells. Cells come from other cells. 2. What are the main differences between prokaryotic and eukaryotic cells? Prokaryote: evolved first on earth, no nucleus, no membrane bound organelles, DNA floats in the cytoplasm in a ring, they are bacteria What parts are present in both? Sometimes a cell wall, cytoplasm, DNA, ribosomes, cell membrane 3. Tell the function of the following organelles: Nucleus-contains the genetic information, control center of the cell Nucleolus-makes ribosomes Mitochondria-cellular respiration, energy conversion Chloroplast-photosynthesis in plants and some bacteria Rough ER-transport Golgi Complex-packaging Lysosome-break down waste and old cell parts Vacuole-storage (support for plant cells) Cell Membrane-support, controls what goes in and out of the cell Cell Wall-protection and support in plant cells and some bacteria Cilia-movement and food capture (small short hair-like structures outside of the cell membrane) Flagella-movement (long whip-like structures outside of the cell membrane) Cell Transport 1. What is passive transport? High concentration to low concentration, cell transport that does not use energy. Includes diffusion, osmosis, and facilitated diffusion. 2. Describe the concentration change in passive transport (HIGH LOW or LOW HIGH?) High to Low 3. What are the three different types of passive transport? Describe each. 1. Diffusion- molecules move from high concentration to low concentration WITHOUT using energy 2. Facilitated diffusion-diffusion of slightly larger or polar molecules with the help of transport proteins. 3. Osmosis- the diffusion of water across a cell membrane 4. What kind of molecules are transported using passive transport? Small/nonpolar 5. What is active transport? Low concentration to high concentration, cell transport that uses energy. 6. What are the four different types of active transport? Describe each. 1. Protein pumps-proteins use energy to pump molecules across the membrane from LOW concentration to HIGH concentration. 2. Endocytosis-the cell brings in large molecules by in-folding its cell membrane 3. Exocytosis- the cell moves out large molecules using its cell membrane 4. Pinocytosis- the moves out liquids using its cell membrane 7. Describe the concentration change in active transport (HIGH LOW or LOW HIGH?) Low to High 8. What kind of molecules are transported using active transport? Large, bulky molecules 9. Describe the structure of the cell membrane. Use terms like hydrophilic, hydrophobic, phospholipid, proteins, … It is made of a bilayer (2) of phospholipids. The heads of the phospholipids are hydrophobic (attract water), while the tails are hydrophobic (repel water). Proteins are embedded within the phospholipids to aid in cell transport. 8. Explain how this structure allows the membrane to be semi-permeable. Small molecules can slip through the phospholipids without any effort by the cell. Larger or charged molecules can be moved through the membrane with the help of proteins. 9. Be able to tell if a cell placed in a solution is hypertonic, hypotonic, or isotonic. Look at your transport study guide and worksheets to practice this. Practice Questions!!!!! Identify the choice that best completes the statement or answers the question. ____ 1. Why is carbon so special compared to other elements? a. Carbon atoms can bond to one another and form a lot of different structures. b. Carbon atoms have four valence electrons and can form quadruple bonds. c. Only carbon atoms can form covalent bonds with oxygen and hydrogen. d. Only carbon atoms can be dissolved in water solutions and suspensions. ____ 2. Amino acid is to protein as a. fat is to lipid. b. DNA is to RNA. c. sugar is to fat. d. simple sugar is to starch. ____ 3. Which of the following is NOT a monomer? a. a glucose molecule b. c. d. an amino acid a nucleotide a protein ____ 4. Which statement is true about macromolecules? a. Simple sugars are made of polysaccharides. b. Glycerol is made of fatty acids. c. Proteins are made of amino acids. d. Nucleotides are made of nucleic acids. ____ 5. A substance that speeds up the rate of a chemical reaction is called a. a catalyst. b. a lipid. c. a molecule. ____ 6. Enzymes affect the reactions in living cells by changing the a. products of the reaction. b. speed of the reaction. c. temperature of the reaction. d. pH of the reaction. ____ 7. Looking at a cell under a microscope, you note that it is a prokaryote. How do you know? a. The cell lacks cytoplasm. b. The cell lacks a cell membrane. c. The cell lacks a nucleus. d. The cell lacks genetic material. ____ 8.. Which organelle breaks down organelles that are no longer useful? a. Golgi apparatus b. lysosome c. endoplasmic reticulum d. mitochondrion ____ 9. Which structure makes proteins using coded instructions that come from the nucleus? a. Golgi apparatus b. mitochondrion c. vacuole d. ribosome ____ 10. Which organelles are involved in energy conversion? a. mitochondria and chloroplasts b. mitochondria and ribosomes c. smooth and rough endoplasmic reticulum d. Golgi apparatus and chloroplasts ____ 11. Unlike the cell membrane, the cell wall is a. b. c. d. found in all organisms. composed of a lipid bilayer. selectively permeable. a rigid structure. ____ 12. Diffusion occurs because a. molecules are attracted to one another. b. molecules constantly move and collide with each other. c. cellular energy forces molecules to collide with each other. d. cellular energy pumps molecules across the cell membrane. ____ 13. During diffusion, when the concentration of molecules on both sides of a membrane is the same, the molecules will a. move across the membrane to the outside of the cell. b. stop moving across the membrane. c. continue to move across the membrane in both directions. d. move across the membrane to the inside of the cell. ____ 14. Which means of particle transport requires input of energy from the cell? a. diffusion b. osmosis c. facilitated diffusion d. active transport ____ 15. What three materials make up many viruses? a. proteins, nucleic acids, and lipids b. capsids, crystals, and nitrogen c. bacteriophages, capsids, and membranes d. RNA, DNA, and bacteriophages ____ 16. The instructions for making new copies of a virus are a. part of a virus’s capsid. b. coded in proteins on the surface membrane. c. coded in either RNA or DNA. d. found only in bacteriophages. ____ 17. Which of the following characteristics of living things is NOT true about viruses? a. contain genetic material b. evolve over time c. obtain and use energy d. able to reproduce ____ 18. If an atom contains 3 protons, 4 neutrons, and 3 electrons, its mass number is a. 3. b. 4. c. 7. d. 11. 19. A plant cell is placed in a solution whose solute concentration is twice as great as the concentration of the cell cytoplasm. The cell membrane is selectively permeable, allowing water but not the solutes to pass through. What will happen to the cell? a. No change b. The cell will shrivel because of osmosis. c. The cell will swell because of osmosis. d. The cell will shrivel because of active transport of water. e. The cell will swell because of active transport of water. ____ ____ 20. Which of the following is a function of the cytoskeleton? a. helps a cell keep its shape b. contains DNA c. surrounds the cell d. helps make proteins 21. The diffusion of water across a selectively permeable membrane is called a. osmotic pressure. b. osmosis. c. pinocytosis. d. active transport. ____ 22. An animal cell that is surrounded by fresh water will burst because the osmotic pressure causes a. water to move into the cell. b. water to move out of the cell. c. solutes to move into the cell. d. solutes to move out of the cell. ____ 23. Which means of particle transport is shown in Figure 7–5 above? a. endocytosis b. exocytosis c. facilitated diffusion d. protein pump _____24. The paramecium's cilia help the organism a. b. c. d. form a shell. control internal water levels. exchange genetic material. swim and capture food. 25. a. b. c. d. Which of the following solutions has the highest H ion concentration? a solution with a pH of 1 a solution with a pH of 4 a solution with a pH of 7 a solution with a pH of 10 Both animal 26. a. b. c. d. Both animal fats and plant oils are made up of glycerol and phospholipids. fatty acids. polar molecules. saturated fats. 27. Which set of atoms are characteristic of nucleic acids? A. C, H, O B. C, H, O, P C. C, H, O, N, S D. C, H, O, N, P Looking at 2 28. a. b. c. d. Looking at a cell under a microscope, you note that it is a prokaryote. How do you know? The cell lacks cytoplasm. The cell lacks a cell membrane. The cell lacks a nucleus. The cell lacks genetic material. 29. Trans 2 29. a. b. c. d. Transport proteins play a role in both passive and active transport. exocytosis and endocytosis. diffusion and vesicle transport. phagocytosis and passive transport. Which pro 3 30. a. b. c. d. Which of the following requires no energy from the cell? exocytosis endocytosis active transport facilitated diffusion