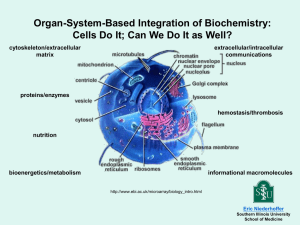
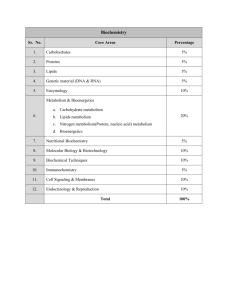
Sample Lecture Syllabus for CHEM222
advertisement

CHEM 222- Introduction to Biochemistry- Fall 2010 Instructor: Didem Vardar-Ulu Time and location: Tue,Wed&Fri 9:50-11:00 AM; SCI-220 Contact Info: SCI-276, x3255 (office), SCI-301, X3285 (lab), dvardar@wellesley.edu Office hours: Tue: 2:30-4:00 pm, Fri: 11:00 – 12:30 pm (or by appointment) Text: Biochemistry 4th edition: Reginald H. Garrett, Charles M. Grisham (Primary) Lehninger Principles of Biochemistry, 4th edition: David L. Nelson, Michael M. Cox (Supplementary) Biochemistry 6th edition: Jeremy M Berg, Lubert Stryer, John L. Tymoczko (Supplementary) Course overview: This course is designed as an introduction to the field of biochemistry. During this single semester course we will focus on developing a strong conceptual understanding for the underlying basis of a wide range of biochemical phenomena by revisiting many concepts that were covered in other chemistry and biology courses. The emphasis will be in demonstrating how these two disciplines can be integrated into a single interdisciplinary approach, to better understand and explain the world we live in. The course is divided into three units: Fundamental building blocks of biochemistry, Enzyme Kinetics/Action, and Medicinal Biochemistry/ Metabolism. Biochemistry is an experimental science: So whenever possible, we will begin our discussions with data drawn from the scientific literature and spend time to analyze and interpret experimental findings. The Laboratory section will be an integral part of the course since essentially all of the material discussed in lecture is the result of empirical biochemical research. Your lab experiments are designed to help you appreciate the difficulties, limitations, and triumphs of “biochemistry at work”. Biochemistry is a collaborative enterprise: So I will encourage and ask you to work in groups on problems and exercises in and out of class. I have seen that group discussions are extremely helpful to everyone in clarifying concepts that may be confusing when you study them on your own. Therefore, there will also be optional weekly opportunities to have brainstorming sessions facilitated by an attached tutor for the course. You are NEVER in competition with one another in my class. You are always evaluated against an absolute standard of excellence. Biochemistry is an interdisciplinary field: So, it will require you to master a new language to describe certain familiar or not so familiar concepts. Becoming fluent in any language is challenging, and takes different amounts of time and effort for each individual. However, it is very rewarding as it opens up a whole new channel of communication. Goals for the course: 1. To help you master enough conceptual understanding of biochemistry so that you can read through, understand, and discuss a current problem in biochemistry at a fundamental level. - Identify the main constituents of a given biological system and describe their physicochemical properties. - Establish a biochemically consistent relationship between the observed function of a given biological system and the structure of its constituents. - Use a multitude of available resources (published literature, online tools, current instrumentation, etc.) to address and provide a plausible answer to a biochemical problem. - Critically analyze a biochemical finding and evaluate the scientific validity of a biochemical explanation. 2. To help you develop customized studying and learning techniques that would help you identify and investigate any new problem in biochemistry. 1 Course conferences: We will be testing out the new Sakai Platform this semester. I will be posting all the assignments and any supplementary material on our Sakai Course site. We will also use this platform for any announcements, so please check it out regularly. Evaluation of work: You will have different kinds of opportunities to demonstrate your learning throughout the semester. Each work will be graded out of 100 and will be weighed according to the following rubric at the end of the semester to determine the letter grade that reflects your overall performance in the course. 1. Concept Checks (6 total, best 5 will be averaged): 10%, 2. Assignments: (Two activities and one problem set) 15% 3. Midterms (2): 15% each (Non-cumulative: scheduled for 10/26/10 and 12/1/10) 4. Final: 20% (self scheduled – comprehensive) 5. Laboratory: 25% (A passing grade of 50% in the lab is required to pass the course) Extra Help: I have included several suggested end of chapter questions (the detailed solutions manual for all questions are at the library). I will also be posting OPTIONAL additional study questions (and answers) for anyone interested in extra practice. I will be available during office hours and by appointment. I am also happy to help out as much as I can via email. I also strongly encourage you to form study groups and hold weekly brain storming sessions where you can discuss lecture and lab material. Based on past experience, studying this material in groups greatly facilitates your learning. Finally, feel free to make use of the tutoring services offered through PLTC or the chemistry help room. Suggestions: I highly value and appreciate any suggestion and constructive criticism you might have regarding any aspect of this course. So please feel free to stop by or send me an email with any suggestions, any time Additional information: Please let me know immediately if you foresee any conflict of important dates due to any personal or religious reasons. I will be happy to consider making any necessary changes or accommodations in advance. Students with disabilities who are taking this course and who need disability-related accommodations are encouraged to work with Verónica Darer, the Directory of Programs of the Pforzheimer Learning and Teaching Center (for learning or attention disabilities), and Jim Wice, the Director of Disability Services (for physical disabilities) to arrange these accommodations. Their offices are in the Pforzheimer Learning and Teaching Center in Clapp Library. 2 CHEM222-F10 Lecture Schedule Lecture 1 2 Date 09/07/10(T) 09/08/10 (W) 3 09/10/10(F) 4 09/14/10(T) 5 6 7 09/15/10 (W) 09/17/10(F) 09/21/10(T) 8 9 10 11 12 13 09/22/10 (W) 09/24/10(F) 09/28/10(T) 09/29/10 (W) 10/1/10(F) 10/5/10(T) Topic Getting to know each other and Introduction to Biochemistry Review of fundamentals – Intermolecular Interactions/ Acids and Bases/Thermodynamics Amino acids, peptides FUNDAMENTAL BUILDING BLOCKS OF BIOCHEMISTRY Proteins I Accessing Biochemical Information: Part 1: Literature search Workshop IPT1 – Amino acids, peptides Proteins II Proteins III Online biochemical information tools: Part 2: Bioinformatics Workshop IPT2 – Proteins, understanding structure/ physicochemical basics Proteins IV Carbohydrates I IPT 3– Proteins, understanding how structure relates to function Carbohydrates II Nucleic Acids I 14 15 16 17 18 19 10/6/10(W) 10/08/10(F) 10/12/10(T) 10/13/10(W) 10/15/10(F) 10/19/10(T) 10/20/10(W) IPT4- Carbohydrates Nucleic Acids II (Monday schedule after Fall Break) IPT5- Nucleic Acids Lipids I Lipids II (membranes) IPT6 - Lipids 20 21 10/22/10(F) 10/26/10(T) 22 23 24 25 10/27/10(W) 10/29/10(F) 11/2/10(T) 11/3/10(W) 26 27 28 29 11/5/10(F) 11/9/10(T) 11/10/10(W) 11/12/10(F) 30 11/16/10(T) Macromolecular Assemblies group presentations MIDTERM I ENZYMES Tanner Conference Enzyme kinetics I Enzyme kinetics II IPT 7– Understanding Enzyme Kinetics (Work on interpreting tables & figures containing enzyme kinetics parameters kcat, Km, Vmax, etc.) Mona Hall Enzyme inhibition and regulation Ligand binding proteins—myoglobin and hemoglobin IPT 8– Enzyme Mechanisms Guest Lecturer: Prof. Dora Carrico-Moniz Cooperativity and allostery in ligand binding proteins MEDICINAL BIOCHEMISTRY/ METABOLISM Bioenergetics 31 11/17/10(W) 32 11/19/10(F) IPT 9– Guest Lecturer: Prof. Adele Wolfson (Thimet oligopeptidase) and problems on coupled reactions/ bioenergetics Bioenergetics/Introduction to metabolism 33 11/23/10(T) Carbohydrate Metabolism I 34 11/24/10(W) 11/26/10(F) No class Thanksgiving 35 36 37 38 39 40 11/30/10(T) 12/1/10(W) 12/3/10(F) 12/7/10(T) 12/8/10(W) 12/10/10(F) Self Scheduled * This Chapter is from Reading Ch 1-2 Ch 1-2 Ch 4 4, 5, 6, 7, 11, 13, 16, 17 Ch 5 1, 5, 8 (use Table 5.2), 14, 16 Ch 6 1, 2, 5, 7, 9, 13, 14, 15 Ch 7 2, 3, 4, 6, 8, 12, 19, 20 Ch 7 Ch 10 Ch 11 3 Activity 1 due 1, 2, 4, 5, 6, 18 DNA movie night 3, 4, 5, 8, 10, 12, 13, 19 Ch 8 Ch 8-9 Ch8: 1, 2, 10, 12, 17, 19, 20, 22 Ch9: 1, 2, 4, 5, 7, 13, 14, 17, 23 Activity 2 due Ch 13 Ch 13,15 Ch 13: 1, 2, 4, 5, 10, 12, 16 Ch 15: 2 Ch 13, 15 Ch 5.1* Ch. 14 Ch 5.1* Ch 14: 1, 2a-b Ch 15: 5, 7, 13, 14 Ch 3 Ch 3 Ch 3 , 17 Ch 18 Carbohydrate Metabolism II MIDTERM II Basics of Metabolic Regulation Tricarboxylic Acid Cycle Electron Transport Chain and Oxidative Phosphorylation Discussion on Medical Myths/ Literature examples; Q&A Final Exam “Lehninger Principles of Biochemistry” Cox Nelson, 2008 Chapter Problems Ch1: 1,3,5,16,17 Ch2: 1-9, 11, 14, 10, 12, 13, 17, 19, 21, 24 Ch22 Ch 15 * Ch 19 Ch 20 Ch3: 1, 2, 3, 5, 6, 9, 12, 14, 17, 20 Ch 17: 4, 6, 7, 8 Problem Set due Ch 18: 1, 8, 9, 10, 12, 13, 22 Ch 22: 2, 4, 7, 8