Formations of Solutions Guide
advertisement

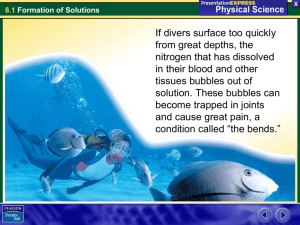
Formation of Solutions Guide Dissolving into Solution Solutions, liquids and1 _______ can be part of a solution. A substance whose particles are dissolved in solution is called a 2_____________. The substance that dissolves a solute is called a 3 _______________. What is the solvent in seawater? 4 ___________ What is the solute in the air we breathe? 5 __________ What is the solvent in the air we breathe? 6___________ A solute will take on the same state of matter as the solvent. Salt is a 7_________ but it becomes a liquid when it is dissolved in the solvent, water. Dispersion Dispersion is the 14___________ apart into smaller pieces and the spreading of those pieces throughout the solution. Sugar and Water are 15___________ molecules. Water molecules constantly 16__________ with the surface of the sugar crystal. 17____________________ form between the water and the sugar molecules. When enough water molecules surround the sugar crystal, it breaks free and is pulled into 18____________ by the water molecules. This exposes another layer of the sugar crystals and the process is repeated. Ionization of Molecular Compounds The dissolving of HCl in water is an example of ionization. Each hydrogen proton combines with water to form a 19_______________ ion (H3O+) and a Cl- ion. The process in which neutral molecules gain or lose electrons and form charged atoms called 20_______, is known as 21______________. This is the only dissolving process that is a 22__________ change. Raising the Boiling Point Ethylene glycol, C2H6O2 added to water is used as a 32___________ in most car radiators. It prevents the engine from overheating by raising the boiling point. It also 33__________ the freezing point of water so that the coolant does not freeze during spells of cold weather. Heat of Solution Breaking the attractions between a solute’s particles and also between the particles of the solvent requires energy so it is an 34____________ process. The formation of new attractions between the solute and the solvent releases energy so it is an 35________________ process. It is the difference in energy between these two processes that is called the 36_________ of solution. The Bends What dissolves in the blood and tissues of a diver because of the pressure as he/she descends? 8 _________________ Why is the bends so painful? 9______________________ Dissolving in Water What are the three ways in which things dissolve in water? 10________________________________________ The attractions holding together the solute and also those of the solvent must be overcome so that they will become attracted to 11________________________. Why is a polar molecule attracted to ions (charged atoms)? 12______________________________________________ The process by which water molecules pull apart the ions of a solute is called 13__________________________ . Properties of Liquid Solutions A solution can differ from its solute or its 23___________ in three physical properties, conductivity, freezing and 24_____________ points. Solid sodium chloride does not conduct electricity but in a solution of sodium chloride and water, it does connect electricity well. Hydrogen chloride gas does not conduct electricity but dissolved in water it does conduct 25____________ well. Lowering the Freezing Point Magnesium chloride (MgCl2) is used for 26 ____________ streets during snowy winter weather. It dissociates into 27______ and 28________ ions. In order for ice to form, water molecules arrange themselves in a 29_____________ structures. Freezing point is lowered by Mg and Cl ions dissolving in water interfering and disrupting the formation of 30____________________. It can lower the freezing point of water as low as 31_________. An Exothermic Process What happen when sodium hydroxide dissolves in water? 37 _____________________. This energy is released when new 38_____________ form. How much is its heat of solution? 39_________. In the whole this is an exothermic reaction because more energy is released than what is needed to 40 ___________ the attractions between solute and solvent. Why Cold Packs? An Endothermic Solution Solid ammonium nitrate (NH4NO3) powder is in the outer bag of a 41 _______________. What is in the inner bag? 42__________ Would it require more energy to break the original attractions than it did to form new attractions? 43_________ A cold pack removes 44__________ from the energy and decreases the size of capillaries. It reduces 45__________ and 46_____________.