CH 2 Questions Level 2 3
advertisement

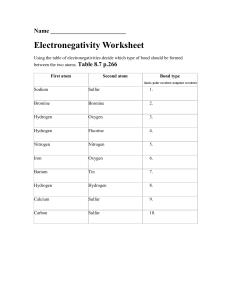
CHAPTER 2 Level 2 and 3 Questions NAME_________________________________________ Level 2 – Answers to these questions are not directly stated by the authors. You must “read between the lines” to answer them. 1. Helium has an atomic number of 2 and atomic mass of 4. Explain. 2. Sketch six molecules of water, indicate their polarity, and how H bonds will form between them. 3. Explain why a hydrogen atom will usually participate in only one covalent bond, while carbon usually participates in four. 4. Compare magnesium + chlorine (two elements with a big difference in electronegativity) with carbon + nitrogen (two elements with a somewhat smaller difference in electronegativity between them). Which pair is most likely to form an ionic bond? Why? What type of bond is the other pair most likely to form? 5. Explain the difference between a hydrogen bond and a covalent bond in which one of the atoms happens to be a hydrogen. CHAPTER 2 Level 2 and 3 Questions NAME_________________________________________ Level 3 – These questions ask you to go farther and read beyond the lines. 6. Why does an oxygen atom form a polar covalent bond when paired with a hydrogen atom, and a nonpolar covalent bond when paired with a nitrogen atom? 7. Why isn’t CO2 bent and polar like H2O? 8. Why is H2O much more likely to participate in chemical reactions than CO2? 9. Is liquid CO2 (yes, it does exist) likely to have a high heat of vaporization like water does? Why or why not? How about CO2’s cohesion capability compared to water’s? (Chapter 3 discusses heat of vaporization and cohesion.) 10. Study the picture. Explain why the electrons in carbon dioxide and water have much less energy than those in methane and O2.