Water Quiz blank
advertisement

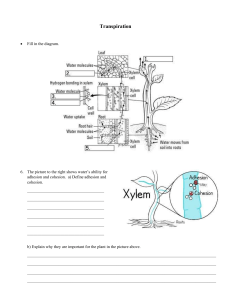
Name: _________________________________ Water Please select the best answer from the choices below _____ 1. Water forms as the result of a/n a. Ionic bond b. Covalent bond c. Metallic bond d. Sulfur bond _____ 2. Which of the following is not a property of water? a. pH regulation b. Adhesion c. Cohesion d. High specific heat _____ 3. a. b. c. d. Water is polar, with the hydrogen side being slightly negative and the oxygen side being slightly positive both sides being slightly positive both sides being slightly negative the oxygen side being slightly negative and the hydrogen side being slightly positive _____ 4. When molecules of water stick together, this is called a. Cohesion b. Surface tension c. Adhesion d. High specific heat _____ 5. The meniscus on a graduated cylinder is the result of a. Cohesion b. Surface tension c. Adhesion d. High specific heat _____ 6. Which of the following elements is not a key element for organisms? a. Fluorine c. Hydrogen b. Oxygen d. Carbon _____ 7. The human body contains approximately 70% water by weight. Water is found inside and outside of cells, and it is able to carry nutrients into and around cells in addition to carrying wastes away from cells. Why is water able to do this? A. Water is a nonpolar covalent compound. B. Water is an ionic solution. C. Water is able to dissolve many substances. D. Water is very acidic. _____ 8. Water is the most abundant molecule found in living organisms. Most mammals, in fact, are approximately 70% water by weight. About two-thirds of this water is present inside cells. The other one-third is present outside cells (e.g., in blood plasma or other body fluids). Why is water so important to cells? A. Almost all the chemical reactions in life processes occur in solutions with water. B. Water determines which proteins are translated from the cellular DNA. C. Water is stored in the cells to be used when the organism gets thirsty. D. The main structural component found in plasma membranes and cell walls is water. ____ 9. In order to change from a solid to a liquid or a liquid to a gas, water must absorb a large amount of heat energy. Water can absorb a large amount of energy while producing only small changes in temperature because water has a A. high specific heat. B. low particle density. C. high particle density. D. low specific heat.