SDS-PAGE
advertisement

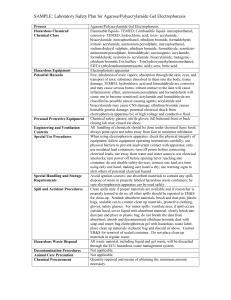
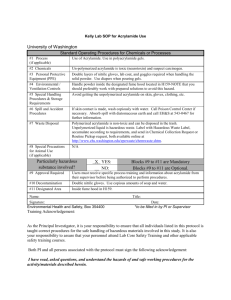
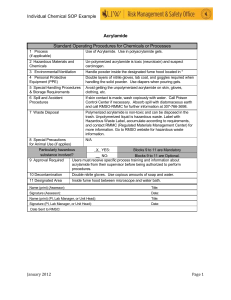
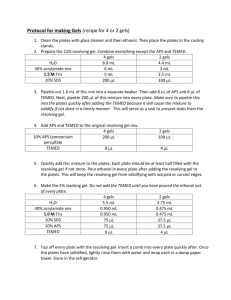
STANDARD OPERATING PROCEDURE Procedure: SDS-PAGE School/Department: SOP prepared by: School of Biological Sciences Nick Coleman (SMB) edit M Joseph Section 1 - Personal Protective Equipment 1. 2. 3. 4. 5. 6. Lab coat or lab gown Nitrile or latex gloves Safety glasses at stages where squirt/splash/spray risk is present Proper enclosed footwear Hair tied back if long Dust mask when handling SDS powder Section 2 – Potential Hazards + Safety precautions 1. Before you begin, consider if pre-cast gels are commercially available for your procedure. These gels reduce many of the hazards mentioned below. 2. Acrylamide used to make gel. Potential for inhalation, skin/eye contact or ingestion with harmful effects. Acrylamide is a potent neurotoxin.! Wear gloves! 3. SDS is harmful by inhalation, ingestion, skin or eye contact. SDS is an irritant. Wear gloves & dust mask when handling powder. 4. TEMED used for making gel. Potential for inhalation, ingestion, skin or eye contact with harmful effects. TEMED produces a strong stench and is a corrosive alkali. TEMED is also flammable. Wear PPE as above. 5. APS used for making gel. Potential for inhalation, ingestion, skin or eye contact with harmful effects. APS is a strong oxidizer and is corrosive. Wear PPE as above. 6. Mercaptoethanol in the buffer used for denaturing protein samples. Potential for inhalation, ingestion, skin or eye contact with harmful effects. Mercaptoethanol produces a strong stench, and is toxic. Wear PPE as above, and handle concentrated mercaptoethanol in the fume hood. 7. Electrophoresis. Potential for serious electrical shock or electrocution due to leaking chamber, faulty or corroded electrode cables, or faulty power supply. Ensure all equipment is in good working order, and that lid is always on electrophoresis tank when in operation. 8. Protein samples. May be recombinant in nature and/or may have other hazardous properties (eg. toxins). Follow approproiate guidelines and work in a PC2 lab if required. 9. Chipped glass plates. Potential for causing lacerations or deep cuts to skin. May also result in leaking of unpolymerised acrylamide solution (refer to above for hazard description). Discard any chipped or damaged plates. 10. Loading samples via needle syringe. Potential for needle stick injury. Handle with care, do not re-sheathe needles. 11. Dispensing Methanol (present in staining and destaining solutions). Potential for exposure via inhalation and direct contact with eyes and skin. May be readily absorbed through skin with possible harmful effects. Methanol primarily affects the central nervous system with symptoms of headache, nausea, and dizziness. Potential fire hazard. Wear PPE, dispose of as hazardous waste. 12. Dispensing Acetic Acid: (present in staining and destaining solutions) Potential for exposure to highly corrosive vapours, and for direct contact of corrosive solution with eyes and skin. May result in irritation, redness and pain, burning and Issue date: 20/11/12 Review date: 21/11/13 Page 1 of 4 ulceration. Wear PPE. Section 3 – Procedure 1. Read and understand this SOP and the risk assessment for SDS-PAGE, along with any MSDS sheets 2. Put on PPE as described above 3. Know the location of spill kits, eyewashes, safety showers before starting work 4. ONLY purchase and use pre-made acrylamide solutions - this removes the significant hazard of inhaling the toxic powder form. 5. Avoid ALL contact with acrylamide solutions or polyacrylamide gels. - do not handle gels without gloves on ! 6. LIMIT acrylamide work to designated areas where possible. Coat work bench with absorbent bench paper prior to working with acrylamide to prevent contamination of non-disposable surfaces. Change this paper layer frequently (eg. weekly or monthly, depending on work intensity) 7. USE barrier/filter tips or disposable plastic transfer pipettes when aliquoting acrylamide solutions to help prevent contamination of pipettes. 8. Solutions containing acrylamide, and the resultant polyacrylamide gels must be disposed of as hazardous chemical wastes to room 225, not down the sink 9. Dispense TEMED, mercaptoethanol and glacial acetic acid in fume hood to minimize stench. Keep containers tightly closed. Keep ignition sources well clear of TEMED vapours – 3 metres is advisable. Do not inhale TEMED, mercaptoethanol or acetic acid vapours, or allow contact with eyes or skin. 10. Inspect electrodes and cables for defects prior to use. DON’T use any electrophoresis equipment that you suspect has an electrical fault or is damaged – in this case, report item to supervisor/ lab manager/ safety officer for electrical testing, repair or replacement. Ensure electrophoresis chamber has a secure LID which prevents accidental contact with the electrified buffer solution 11. If the protein samples have come from recombinant organisms or clinical specimens, these need to be handled in a PC2 lab, using PC2 methods, and any wastes disposed of according to PC2 guidelines (see related risk assessments on risk group 2 microbes and clinical samples). Protein samples may have other associated risks eg. toxins – always handle with gloves, and dispose of excess via autoclaving or similar (not down the sink). Know the details and potential risks of the proteins you are working on before you start handling them. 12. Use containers with secure lids when staining and destaining gels, and decant solutions slowly and carefully to prevent splashing. 13. Ensure area around electrophoresis chamber is free of spills or leaks PRIOR and DURING the running of your gel 14. For detailed procedure for making and running SDS-PAGE gels, consult instruction manual of electrophoresis apparatus, or practical manual from appropriate course. Section 4 – Disposal / Spills / Incidents 1. Depending on the nature of the spill, clean up as described in SOPs for Biohazard Spills, Flammables, Corrosives, or Toxic Substances. Any acrylamide spills must be cleaned up immediately and thoroughly using first dry paper towel, then wet paper towel, and the towels disposed as hazardous waste. 2. Depending on the nature of the materials used, dispose of wastes as described Issue date: 20/11/12 Review date: 21/11/13 Page 2 of 4 in SOPs for Biohazard Spills, or Chemical Wastes 3. Any large spills of hazardous materials (>1L) or incidents resulting in injury must be reported to your supervisor immediately and via the online incident report form within 24 h. Near misses (dangerous situations not leading to an incident) should also be reported Section 5 – Repairs / Certification / Validation 1. Ensure electrophoresis equipment is kept in good repair. Consult the manufacturer or the SMB service centre if in doubt Section 6 – Relevant Material safety data sheets 1. Consult MSDS for acrylamide, TEMED, APS, SDS, acetic acid Section 7 - References 1. 2. Essential: Risk assessment for SDS-PAGE As needed: Risk assessment and SOP for Flammables, Toxic chemicals, Disposing of chemical wastes SOP Training Confirmation By signing below, these individuals confirm that they have read and understood the SOP, and agree to always follow the instructions in this SOP when performing this procedure. Position Supervisor employee / student employee / student employee / student employee / student employee / student employee / student employee / student employee / student employee / student employee / student employee / student employee / student employee / student employee / student employee / student employee / student employee / student employee / student Name Signature Date WHS Committee Approval Representative: A. Prof Frank Seebacher Chair Safety Committee Signature: ........................................................ Date: ..................................... Issue date: 20/11/12 Review date: 21/11/13 Page 3 of 4