38_RA_MyHC Gel electrophoresis_CL
advertisement

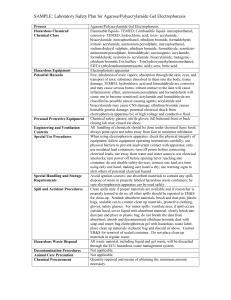
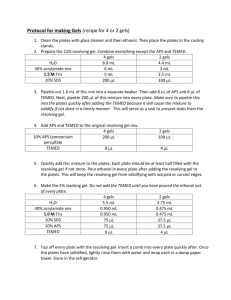
OHS017 OHS Risk Assessment and Control Form Risk assessment completed by: Dr C.A. Lucas Staff/student number:3283496 Faculty/Division: Medicine Document number SOMS.CGM.RA038 School/Unit: School of Medical Sciences, Oncology Research Units Initial Issue date 30/06/09 Current version 1.0 Current Version Issue date 30/06/09 Next review date 30/06/12 For additional information refer to the OHS Risk Assessment and Control Procedure, the OHS Risk Rating Procedure and the Hierarchy of Risk Controls. Risk Assessment title: High Resolution MyHC gel electrophoresis Step 1: Identify the activity Describe the activity: The task involves setting and running high resolution SDS gel electrophoresis to specifically separate Myosin Heavy Chain isoforms . This involves the pouring of bis-acrylamide stock solution containing Tris buffer, glycerol, EDTA, glycine, sodium dodecyl sulphate, ammonium persulphate, and TEMED to make polyacrylamide gels for use in high resolution SDS gels. TEMED and ammonium persulphate catalyse the polymerization reaction of Bis-acrylamide –acrylamide to form a gel matrix which is used to separate MyHC isoforms. For detailed protocols refer to MyHC gel electrophoresis SWP Describe the location: Electrophoresis performed in Rm 501 Step 2: Identify who may be at risk by the activity A number of people may be at risk from any activity. This may affect the risk controls needed. These people may include fellow workers, visitors, contractors and the public. The location of the activity may affect the number of people at risk. The operator, fellow workers, visitors and contractors __________________________________________________________________________________________________________________________________________________________________________ _________ Page 1 of 6 Risk Assessment and Control Form Date Effective: 01/01/2007 Uncontrolled document when printed Current Version: 2.6, 16/07/2008 Steps 3 to 7: Identify the hazards, risks, and rate the risks 1. An activity may be divided into tasks. For each task identify the hazards and associated risks. 2. List existing risk controls and determine a risk rating using the UNSW Risk Rating Procedure. 3. Additional risk controls may be required to achieve an acceptable level of risk. Re-rate the risk if additional risk controls used. Tasks Hazards Associated risks (Step 3) (Step 4) Risk rating with existing controls * Additional risk controls required Risk Rating with additional controls * (Step 5) (Step 6) (Step 7) Existing risk controls C Preparation of Protein samples for MyHC SDS gels Pouring Acrylamide gels Biological exposure to protein samples, Bromophenol blue, DTT Causes irritation to respiratory system, eyes and skin upon inhalation and contact Use standard PPE which include laboratory coat, Nitrile gloves and safety glasses when handling biological samples and chemicals 2 Leaking gel cassettes. Absorption of acrylamide through skin. Acrylamide affects central and peripheral Nervous System and reproductive system when swallowed, inhaled or absorbed through skin PPE: Gown, Nitrile gloves, safety goggles, enclosed shoes. 2 L D R L (Apply the hierarchy of risk controls) Use face mask. C L R 2 E L 2 E L Read MSDS prior to the usage ofchemicals Spill kit Hazardous substances training course D L Use face mask. Read MSDS prior to the usage ofchemicals Spill kit Hazardous substances training course __________________________________________________________________________________________________________________________________________________________________________ _________ Page 2 of 6 Risk Assessment and Control Form Date Effective: 01/01/2007 Uncontrolled document when printed Current Version: 2.6, 16/07/2008 Pipetting liquidsTEMED Setting up and running of gel electrophoresis TEMED is toxic via inhalation or absorption. TEMED is flammable and corrosive. Toxicity associated with ihalation or absorption through skin. Corrosive skin burns by TEMED contact TEMED solutions kept away from ignition sources and naked flames in the lab Electrical Hazard Electrical Shock Chemical exposure to electrophoresis buffer SDS electrophoresis buffer may cause irritation to eyes and skin upon contact Equipment manufactured to Australian Standards 3 D M TEMED decanting/pipetting into solutions to be done in a fume hood 2 D L 2 D L Place electrophoresis power supply on a elevated position separated from electrophoresis tank 2 E L PPE: Gown, Nitrile Gloves, enclosed shoes Ensure power supply pack is turned off when connecting cables Ensure SDS electrophoresis buffer is not filled beyond max fill line Conduct visual check on equipment prior to use Equipment is tagged periodically Standard PPE when loading gel cassettes into the electrophoresis system __________________________________________________________________________________________________________________________________________________________________________ _________ Page 3 of 6 Risk Assessment and Control Form Date Effective: 01/01/2007 Uncontrolled document when printed Current Version: 2.6, 16/07/2008 Disposing of gels Acrylamide is highly toxic. Acrylamide can be absorbed through skin and is a carcinogen and neurotoxin. Overexposure may lead to health disorders. PPE: Gown, Nitrile gloves, enclosed shoes. 2 E L Acrylamide gels disposed of as cytotoxic waste * C = consequence L = likelihood R = risk rating from the UNSW Risk Rating Procedure Step 8 Documentation and supervisor approval Completed by: Christine Lucas (signature) Authorised by: Edna Hardeman (signature) Date: Jun09 Step 9: Implement the additional risk controls identified Indicate briefly what additional risk controls from Step 6 above were implemented, when and by whom. Risk control: Date: Implemented by: Risk control: Date: Implemented by: Step 10: Monitor and review the risk controls It is important to monitor risk controls and review risk assessments regularly. Review is required when there is a change in the process, relevant legal changes, and where a cause for concern has arisen. Reviews could be scheduled on an annual basis. If the risk assessment has substantially changed a new risk assessment is warranted. Review date: Reviewed by: Authorised by: Review date: Reviewed by: Authorised by: __________________________________________________________________________________________________________________________________________________________________________ _________ Page 4 of 6 Risk Assessment and Control Form Date Effective: 01/01/2007 Uncontrolled document when printed Current Version: 2.6, 16/07/2008 Documentation It is a requirement that legal and advisory documentation that supports this risk assessment be listed. Such documentation includes Acts, Regulations, Australian Standards and Codes of Practice, where applicable. NSW OHS Act 2000 NSW OHS Regulation 2001 Safe Work Procedure Form (OHS026) Australia Dangerous Goods Code. AS/NZS 2243.2:2006. Safety in laboratories. Part 2: Chemical aspects Australian Standard AS2243.3-2002. Safety in laboratories. Part 3: Microbiological aspects and containment facilities. AS/NZS 2161.1:2000 Occupational Protective Gloves – Selection, Use and Maintenance AS/NZS 1336:1997 Recommended Practices for Occupational Eye Protection __________________________________________________________________________________________________________________________________________________________________________ _________ Page 5 of 6 Risk Assessment and Control Form Date Effective: 01/01/2007 Uncontrolled document when printed Current Version: 2.6, 16/07/2008 UNSW Concise OHS Risk Rating Table OHS697 What you need to do 1. Consider what can go wrong that can hurt someone 2. Determine what the most likely outcome would be - Consequences 3. Determine how likely those consequences are - Likelihood 4. Calculate the risk rating 5. Required action How severely could someone be hurt death or permanent disability to one or more persons hospital admission required medical treatment required first aid required injuries not requiring first aid CONSEQUENCES: Severe Major Moderate Minor Insignificant How likely are those consequences? expected to occur in most circumstances will probably occur in most circumstances could occur at some time is not likely to occur in normal circumstances may occur only in exceptional circumstances LIKELIHOOD: Almost certain Likely Possible Unlikely Rare CONSEQUENCES Insignificant 1 Minor 2 Moderate 3 Major 4 Severe 5 M H H VH VH M M H H VH Possible C L M H H VH Unlikely D L L M M H Rare E L L M M M LIKELIHOOD Almost certain A Likely B Risk level Very high High Medium Low Required action Act immediately: The proposed task or process activity must not proceed. Steps must be taken to lower the risk level to as low as reasonably practicable using the hierarchy of risk controls. Act today: The proposed activity can only proceed, provided that: (i) the risk level has been reduced to as low as reasonably practicable using the hierarchy of risk controls; (ii) the risk controls must include those identified in legislation, Australian Standards, Codes of Practice etc. (iii) the risk assessment has been reviewed and approved by the Supervisor and (iv) a Safe Working Procedure or Safe Work Method has been prepared. (v) The supervisor must review and document the effectiveness of the implemented risk controls. Act this week: The proposed task or process can proceed, provided that: (i) the risk level has been reduced to as low as reasonably practicable using the hierarchy of risk controls; (ii) the risk assessment has been reviewed and approved by the Supervisor and (iii) a Safe Working Procedure or Safe Work Method has been prepared. Act this month: Managed by local documented routine procedures which must include application of the hierarchy of controls. _______________________________________________________________________________________________________________ Page 6 of 6 UNSW Concise OHS Risk Rating Table Effective date: 01/01/2007 Uncontrolled document when printed Current Version: 2.6,16/07/2008