Evaporation Experiment
advertisement

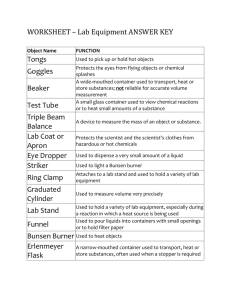
Evaporation Experiment Aim: To separate the different substances by evaporating the filtered water. This is to determine whether there is or is not any other substances within the water. Apparatus: Evaporating basin Tripod Bunsen burner Work mat Matches Filtered water (previous experiment) Method: 1. Set up the apparatus by getting the evaporating basin and putting it on to the tripod. 2. Place Bunsen under tripod and put the safety flame on then transfer it to working flame. 3. Place the Bunsen burner near the edge of the tripod so that the solution dose not boil or overheat over the evaporating basin. Observations: Substances found on the edge of evaporating basin. Substance turn into salt. Results: The substances where separated from the heat. Near the end of the experiment the solution turned to salt. This is because the liquid was heated and evaporated which leaves the salt. Discussion: I believe that it was both a physical and chemical change because it changed state and had a chemical change. The changed of state happened when the water evaporated in to the air and just left salt. The chemical change happened when the carbon dioxide and the H20 disappeared.