jace13734-sup-0001
advertisement

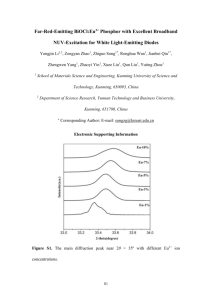
Supporting Information Particle Size and Crystal Phase Dependent Photoluminescence of La2Zr2O7:Eu3+ Nanoparticles Madhab Pokhrel1, Mikhail G. Brik2, 3, 4 and Yuanbing Mao1* 1 Department of Chemistry, University of Texas-Pan American, 1201 West University Drive, Edinburg, Texas 78539, USA 2 College of Sciences, Chongqing University of Posts and Telecommunications, Chongqing 400065, China 3 Institute of Physics, University of Tartu, Ravila 14C, Tartu 50411, Estonia 4 Institute of Physics, Jan Dlugosz University, Armii Krajowej 13/15, PL-42200 Czestochowa, Poland *To whom correspondence should be addressed: Tel: +1 956 665 2417; Fax: +1 956 665 5006; E-mail: maoy@utpa.edu ESI-1 Synthesis of 5 % Eu3+ doped La(OH)3·ZrO(OH)2·nH2O precursor: The starting materials including lanthanum nitrate hexahydrate (La(NO3)3•6H2O, 99.0%), zirconium dinitrate oxide hydrate (ZrO(NO3)2•xH2O, 99.9%), europium (III) nitrate hexahydrate (Eu(NO3)3•6H2O, 99.9%), potassium nitrate (KNO3, 99.9%), sodium nitrate (NaNO3, 98%) and 1|Page ammonia (NH4OH, 28.0−30.0%) were purchased from Sigma Aldrich. All chemicals are of analytical grade reagents and used directly without further purification. In the first step, single-source complex precursors of La(OH)3·ZrO(OH)2:Eu(OH)3·nH2O were prepared via a co-precipitation route. In a typical synthesis process, 4.75 mmol lanthanum nitrate hexahydrate, 5 mmol zirconium dinitrate oxide hydrate, and 0.25 mmol europium (III) nitrate hexahydrate were dissolved in 200 mL of deionized water to form a clear solution. Then a 200 mL of dilute ammonia solution with a specific concentration was slowly dropped into the above metal nitrate solution under magnetical stirring. After further agitation and aging for another 2 hours, the formed white precipitation was collected by centrifuged and washing with deionized water for several times. Finally, the single-source complex precursor of La(OH)3·ZrO(OH)2:Eu(OH)3·nH2O was air dried overnight. Herein, in order to adjust the size of final La2Zr2O7:5%Eu3+ NPs, ammonia solutions with concentrations of 10, 5 and 2.5(v/v) % were used. Synthesis of La2Zr2O7:5%Eu3+ NPs In the second step, La2Zr2O7:5%Eu3+ NPs were synthesized size-controllably through a facile molten salt synthetic process using the single-source complex precursors of La(OH)3·ZrO(OH)2:Eu(OH)3·nH2O prepared above. Typically, 0.35 g of the as-prepared precursor was first ground together with 60 mmol of nitrate mixture (NaNO3:KNO3 = 1:1, molar ratio). After being ground homogenously, the mixture was transferred into a covered ceramic crucible and heated up to 650 ℃ with a rate of 10 ℃/min in a box furnace. After isothermally treated for 6 hours, the sample was cooled down to room temperature at a rate of 10 °C/min. Then the resulting product was washed with copious amount of deionized water and centrifuged for 2|Page collection. After dried in an oven at 120 ℃ overnight, the La2Zr2O7:5%Eu3+ NPs were obtained. Corresponding to the concentrations of the added ammonia solutions, i.e. 10, 5 and 2.5 (v/v) %, for preparing the precursor of La (OH)3·ZrO(OH)2:Eu(OH)3·nH2O, the as-prepared La2Zr2O7:5%Eu3+ NPs were labeled as S1, S2 and S3, respectively. The as-prepared sample S3 was further calcinated at 800 and 1000 ℃ in air for 6 h and noted as S4 and S5, respectively. The total synthetic procedure is summarized schematically as shown in Fig. 1. ESI-2. QY Setup and measurements for the La2Zr2O7:5%Eu3+ NPs All the excitation and emission spectra were corrected for the spectral sensitivity of the system and detector, as well as intensity variation in the Xenon lamp light source using a reference diode. In addition, the temporal evolutions (photoluminescence decay) of the emission intensity as a function of time were measured utilizing a pulsed xenon flash-lamp excitation source. The source has a pulse width of approximately 2 µs and the wavelength was controlled by the excitation monochromater. The collected decay curve was analyzed using Exponential Fit Analysis software F980 provided by Edinburg Instruments. All the emission and excitation spectra measurements were performed at room temperature. For absolute QY measurements, a 150 mm BenFlect coated integrating sphere was employed in the Edinburgh Instruments FLS980 fluorescence spectrometer. Spectral sensitivity for the fluorescence spectrometer and sphere was corrected using a calibrated lamp for spectral light throughput. Powder samples of the as-prepared La2Zr2O7:5%Eu3+ NPs were held in a specially designed sample holder mounted at the middle of the integrating sphere, engineered with baffles 3|Page to ensure light is homogenized before exiting the sphere. Samples were excited at 258 nm using the Xenon lamp light source. To be consistent with all experiments performed, the same sample weight (90 mg) and monochromater slit size (3 nm for both excitation and emission monochromaters) were kept identical for all experiments, i.e. same setup was used to compare fluorescence intensities between samples. Fluorescence spectra in the range of 500-750 nm and excitation spectra of 250-272 nm not absorbed by the sample and reference were collected after diffuse reflectance from the samples relative to a non-absorbing standard at the excitation wavelength and emission spectra under the same condition. To avoid reabsorption, very thin layer of samples was used in all experiments. The fluorescence QY was measured by finding the ratio of the area under the corrected emission spectra to the difference in corrected area under the diffuse reflectance spectra for the samples and the reference.1 The spectra were collected after diffuse reflectance from the samples relative to a non-absorbing standard (BenFlect) at the excitation wavelength and emission spectra under the same condition. The emission spectra of 500-750 nm and excitation spectra of 240-272 nm not absorbed by the samples and reference was measured using TE-cooled photo-multiplier tube (Hamamatsu, Model R928P) as shown in Figure S6. The QY was measured by finding the ratio of the area under the emission spectra to the difference in corrected area under the diffuse reflectance of the excitation spectra for the sample and the reference as shown in equation below. QY Asample NPemitted Nem d , NPabsorbed N absd Areference Asample Including all the possible errors, such as reflectivity of the reference (< 3%), particle size effects (< 2%), and diffuse reflectance from the sample holder (< 3%), we estimated the error in calculated 4|Page QYs is about 10 %. Similarly, we estimated the fluctuation for excitation power is about 2%. The excitation power was measured to be 6 mW. Figure S2. Excitation and emission spectra collected after the diffuse reflectance from the sample and the reference. Emission spectra were multiplied 10 times for better visibility. Table S2. PL QY for sample S5 at RT under 258 nm and 244 nm excitation after integrating the wavelength range from 500 to 750 nm. Samples S5 S5 Excitation Wavelength 258 244 QY (%) 6.01±0.60 5.96±0.59 5|Page ESI-3. Evaluation of the particle size of the La2Zr2O7:5%Eu3+ NPs using Scherrer Equation Profile fitting is the most precise way to determine diffraction peak position, intensity, and width for calculating lattice parameters and crystallite size. Figure S2 shows the Gaussian fitting for sample S1 and S2, and an estimation of FWHM for the XRD peak corresponding to La2Zr2O7:5%Eu3+ centered at 2θ = 28.53 °. The corrected FWHM and the calculation of particle size for all other three samples have also been listed in Table S1. Figure S3. Profile fitting to determine FWHM for calculating crystallite size of the La2Zr2O7:5%Eu3+ NPs: sample S1 (left panel) and sample S2 (right panel). Table S3. The evaluated crystalline size of the La2Zr2O7:5%Eu3+ NPs using Scherrer equation. Samples cos(θ)→θrad FWHM (Rad) D = Kλ/βcosθ, λ = 0.154 nm S1 Θ = (28.53/2)° 0.016 9 nm S2 0.0091 17 nm S3 0.0055 28 nm S4 0.0040 38 nm S5 0.0022 70 nm ESI-4. Comparisions of Raman spectra between samples of S3, S4 and S5 6|Page Figure S4. Raman spectra for La2Zr2O7:5% Eu3+ NP samples of S3, S4 and S5. From the Raman spectra, the strongest band located at ~302 cm−1 can be assigned to the internal La-O stretching mode, the band of 390 cm−1 is attributed to the O-Zr-O stretching. The other bands at 508, 621,730, and 842 are attributed to T2g mode of fluorite structure.2 The La-O stretching mode located at ~302 cm−1 can be used to identify the ordering degree of oxygen vacancies and cations. For samples S3 and S4, the corresponding Raman spectra are boarder that that of sample S5, consistent with the fact of disordered fluorite structure vs pyrochlore structure of the La2Zr2O7:5% Eu3+ NPs by XRD. At the meantime, sample S4 became less disorder after thermal annealing at 800 ℃, as confirmed with sharper Raman peak at ~302 cm−1 compared to that from sample S3. ESI-5. BET Method for Determining Surface Areas for samples of S1, S3, S4 and S5 7|Page Figure S5. (a) Adsorption isotherms for nitrogen in La2Zr2O7:5% Eu3+ NP especially samples of S1, S3, S4 and S5 at 77 K, (b) evaluation of BET surface areas calculated from the standard BET pressure range. ESI-6. Structure and EDX Analysis of the La2Zr2O7:5%Eu3+ NPs In addition, the compositional distributions of each element in the La2Zr2O7:5%Eu3+ NPs were confirmed by Energy Dispersive X-ray (EDX) spectral mapping analysis as shown in Figures S3a and S3b. Spectral mapping was performed in a cluster of particles as shown Figure S3a. EDX signals obtained from K, L, and M shell electrons were clearly traced across the region of the La2Zr2O7:5%Eu3+ NPs as shown in Figure S3a which guarantees the doping of Eu3+ in the La2Zr2O7:5%Eu3+ NPs. 8|Page Figure S6. As-prepared La2Zr2O7:5%Eu3+ sample S3: (a) SEM image, (b) corresponding EDX spectrum showing the chemical composition. Extra peaks in the spectrum, such as aluminum and carbon peaks, correspond to the SEM stub and carbon tape to hold the La2Zr2O7:5%Eu3+ NP sample. 9|Page ESI-7 Purposed energy transfer diagram of the La2Zr2O7:5%Eu3+ NPs under 258 nm excitation Figure S7. A simplified energy level diagram and the proposed energy transfer mechanisms under 258 nm excitation and Eu3+ ions emissions for the La2Zr2O7:5%Eu3+ NPs. 10 | P a g e ESI-8. Shift in charge transfer (CT) band of the La2Zr2O7:5%Eu3+ NPs CT bands corresponding to Eu3+-O2- was located at shorter wavelengths for sample S1 compared to those of S2 and S3. Maximum shift of 1 nm was observed between sample S1 and S3 (Figure S4a, panel a). However CT band corresponding to Eu3+-O2- was located at 244 nm for sample S5 compared to that 258 nm for S3 (Figure S4a, panel b). The reasons for the above differences may be related to crystal structure (fluorite vs. pyrochlore) and order of Eu3+ symmetry in these two crystal phases of the La2Zr2O7 host. Figure S8. (a) Excitation spectra for samples S1 and S3 showing the shift in CTB between the samples prepared with different concentrations of the added ammonia, (b) excitation spectra for sample S3 before and after annealing at 800 and 1000 °C for 6 hrs showing the shift in CTB among the samples S3, S4 and S5. 11 | P a g e ESI-9. Multiexponential fitting for decay of the La2Zr2O7:5%Eu3+ NPs The PL decay of the emission intensity at 612 nm under 258 nm excitation as a function of time was measured utilizing a pulsed xenon flash-lamp excitation source. The entire system was controlled though Edinburgh Instruments F980 data acquisition software. The collected decay curve was analyzed using F980 software provided by Edinburg Instruments. Figure S7 shows the decay monitored for sample S3 at 612 nm emission and its fitting. In addition, fitting parameter, i.e chi-square for each fitting was in the range of 0.95 to 1.2. To quantify the luminescent dynamics, we estimate the effective lifetime (τeff) by using the following equation. eff A1 1 A2 2 A1 1 A2 2 2 2 where A1 and A2 are the fitting parameters and 1 and 2 are the fluorescence decay times Figure S9. Luminescence decay monitored for the La2Zr2O7:Eu3+ NPs at 612 nm under 258 nm excitation. Two exponential functions were used for fitting. 12 | P a g e Table S9. Effective lifetime (τeff) evaluation by fitting the decay curves with a multi exponential function. sample S1 S2 S3 S4 S5 τ1 0.708 0.728 0.764 0.806 0.851 τ2 1.932 1.952 1.973 2.139 2.459 τ1^2 0.501 0.529 0.583 0.649 0.724 τ2^2 3.73 3.81 3.89 4.54 6.00 eff A1 1 A2 2 A1 1 A2 2 2 2 1.79 1.78 1.82 1.93 2.24 ESI-10: Site symmetry Analysis of the La2Zr2O7:5%Eu3+ NPs The spectrum of Eu3+(4f6) in La2Zr2O7 was obtained between 500 nm and 750 nm at room temperature (RT) at different excitation wavelengths as shown in Fig. 7a and 7b for both crystal phase of La2Zr2O7: Eu3+ nanoparticles. The relative similarity both in the spectra and the Stark splitting’s of emission bands was observed both under band (Eu3+ band) and above band (258 nm) excitation as shown Fig. 7a and 7b. This indicates that all the emission in La2Zr2O7: Eu3+ is from Eu3+ single site. The observed number of Stark splittings for the 5D0→7F2 transition at under band (Eu3+ band) excitation is consistent with Eu3+ ions entering a substitutional La site with D3d symmetry as reported in the previous literature. 3 In general, Eu3+ is assumed to replace the site of La3+ in the D3d crystalline field because of the same valence and similar ion radius. In addition, the I612/I592 ratios was always larger than 1 and the 612 nm emission is the most dominating one in both fluorite (Fig. S7 (a) and pyrochlore crystal phases as shown in Fig. S7 (b). Overall similar emission profiles observed in this study ensure that the original cation (La3+) site symmetry (D3d) is preserved. 13 | P a g e Figure S10. PL spectra of (a) sample S3 and (b) sample S5, showing the dependence of the 5 D0→7F1, 5D0→7F2-4 emissions intensity on the excitation wavelengths. Overall similar spectral profile was observed in this study for all Eu3+ band excitation, however PL intensity was found maximum for 258 nm excitation. References 1. M. Pokhrel, G. A. Kumar, and D. K. Sardar, "Highly efficient NIR to NIR and VIS upconversion in Er3+ and Yb3+ doped in M2O2S (M = Gd, La, Y)," Journal of Materials Chemistry A, 1[38] 11595-606 (2013). 2. Y. Tong, S. Zhao, W. Feng, and L. Ma, "A study of Eu-doped La2Zr2O7 nanocrystals prepared by saltassistant combustion synthesis," Journal of Alloys and Compounds, 550[0] 268-72 (2013). 3. K. Holliday, S. Finkeldei, S. Neumeier, C. Walther, D. Bosbach, and T. Stumpf, "TRLFS of Eu3+ and Cm3+ doped La2Zr2O7: A comparison of defect fluorite to pyrochlore structures," Journal of Nuclear Materials, 433[1–3] 479-85 (2013). 14 | P a g e