jace13589-sup-0001-SuppInfo
advertisement
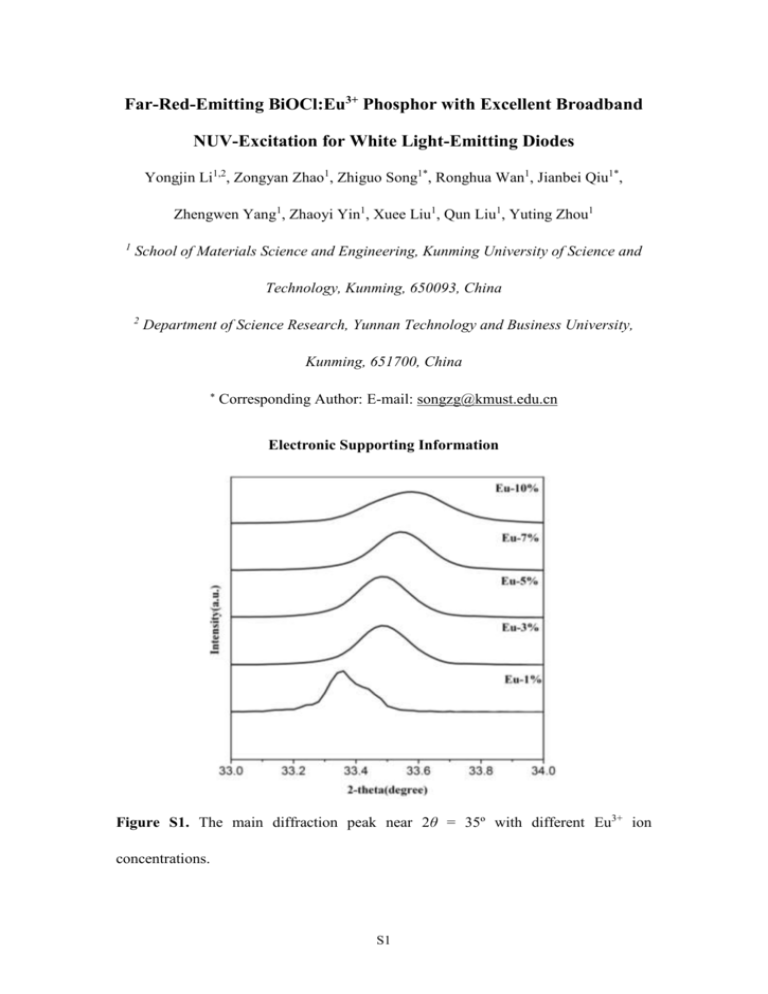
Far-Red-Emitting BiOCl:Eu3+ Phosphor with Excellent Broadband NUV-Excitation for White Light-Emitting Diodes Yongjin Li1,2, Zongyan Zhao1, Zhiguo Song1*, Ronghua Wan1, Jianbei Qiu1*, Zhengwen Yang1, Zhaoyi Yin1, Xuee Liu1, Qun Liu1, Yuting Zhou1 1 School of Materials Science and Engineering, Kunming University of Science and Technology, Kunming, 650093, China 2 Department of Science Research, Yunnan Technology and Business University, Kunming, 651700, China Corresponding Author: E-mail: songzg@kmust.edu.cn Electronic Supporting Information Figure S1. The main diffraction peak near 2θ = 35º with different Eu3+ ion concentrations. S1 Table S1 Cell constants a, b, c, and cell volume (V) of Eu3+-doped BiOCl with different Eu3+ ions concentration Eu3+ concentration (mol%) Lattice constant(Å) a=b c V (Å3) 0 3.898 7.379 112.12 1 3.893 7.356 111.51 3 3.892 7.362 111.52 5 3.891 7.364 111.46 7 3.890 7.350 111.23 10 3.887 7.343 110.96 From the Figure S1, it is noteworthy that the diffraction peaks are shifted to a high 2θ angle with increases Eu3+ ions concentrations, implying that the Eu3+ ions have been doped into the lattices of BiOCl. On the other hand, the diffraction peaks get broadened as the Eu3+ concentration is increased, suggesting a systematic decrease in the grain size. The peaks which correspond to the crystal planes (001) and (110) of the tetragonal phase are selected to calculate the lattice parameters of the Eu3+-doped BiOCl phosphors. Using the Equation S-1, the lattice parameter and unit cell volume of the samples were evaluated (Table S1). 1/d2 = (h2+k2)/a2 + l2/c2 (S-1) Here, h k l are the Miller indices; a, b, and c are the lattice parameters (in a tetragonal system, a = b ≠ c); d is the interplanar spacing between the crystal planes (hkl). As can be seen from the estimated data, the estimated lattice parameters and unit cell volume values for the Eu3+ doped BiOCl deviate considerably from those of the un-doped BiOCl due to the incorporation of Eu3+ ions into the BiOCl lattice. Because S2 the radius of Eu3+ is smaller than that of Bi3+, the lattice constants become smaller while Eu3+ substitutes Bi3+. Figure S2. Diffuse reflectance spectra of Eu3+-doped BiOCl phosphor with different Eu3+ ion concentrations. Band gap energy of samples according to the diffuse reflectance spectra It was reported that the absorption coefficient (α) of a semiconductor and its band gap energy (Egap) are related through the following equation:1,2 αhv = (hvEgap)n (S-2) where h is Planck’s constant, v is the frequency of the light, and n is a constant depending on the transition type, which can be 1/2, 3/2, or 2 corresponding to direct allowed, direct forbidden, or indirect transition, respectively. In our cases, BiOCl has been confirmed to be indirect materials; thus, n = 2. According to the Kubelka-Munk equation:1 F(R∞) = (1 R∞)2/2 R∞ = k/s (S-3) where R∞ is the diffuse reflectance of the layer relative to the standard, k is the molar S3 absorption coefficient of the sample, and s is the scattering coefficient. Function F(R∞) can be obtained from the DR spectra shown in Figure 3(a) and Figure S2. For a given material, s is independent of the wavelength of the incident light, and k is proportional to the in Eq. S-2. As a result, F(R∞) is proportional to the absorption coefficient α; substituting α with F(R∞) in Eq. S-2, we obtain hvF(R∞) (hv Egap) (S-4) By plotting [hvF(R∞)]1/2 ∼hv in panels (b) and (c) of Figure 3, we determined the band gap energies of un-doped BiOCl and 0.07Eu3+ -doped BiOCl to be 3.33 and 2.81 eV, respectively. Additionally, as for samples doped with 0.01, 0.03, 0.05, and 0.1 Eu3+, by the same method, the band gap energies are 3.21, 3.07, 2.98, and 2.71, respectively. Figure S3. (a) Excitation spectrum of Eu3+ (7 mol%)-doped BiOBr phosphor recorded at λem= 622 nm; (b) Diffuse reflectance spectra of Eu3+ (7 mol%)-doped BiOBr phosphor. S4 Figure S4. Emission spectra of Eu3+ (7 mol%)-doped BiOCl phosphor exciting at 466 nm. Theoretical calculation of charge transfer (CT) Method 1: The charge transfer excitation of Eu3+ is an electronic transition from the ground-state to the excited-state of the 4f shell, and the corresponding band position in excitation spectrum is mainly determined by the covalency of Eu-O bond and the coordination environment of Eu3+ as well. As usual, an increase in covalency of Eu-O bond would reduce the energy for electron transfer. In the Eu-O couple, the excitation energy for charge transfer can be estimated by the following Jorgensen’s equation,3 ν=3[χ(O)- χ(Eu)] × 104 (S-5) where ν in cm-1 denotes the position of the charge transfer band, χ(O) and χ(Eu) are the optical electronegativity of the oxygen and europium ions, respectively. Taking χ(O)= 3.2 and χ(Eu) =1.75, the band position for Eu-O charge transfer is calculated to be at 230 nm (or 43500 cm-1) in BiOCl. S5 Method 2: According to the equation:4 ECT = 2.542 + 0.171 ×Eμg (S-6) where ECT is CT energy, and Eμg is average energy gap for any μ bond in units of electron volts (eV). Because YOCl, LaOCl and BiOCl all have tetragonal PbFCl-type structure (space group P4/nmm; No. 129), moreover, the radius is Y3+< Bi3+< La3+. Therefore, we reason that Eμg of BiOCl (Bi-Cl) is between YOCl (Y-Cl) and LaOCl (La-Cl). According to Ref. 5 and Eq.S-6, the ECT of YOCl and LaOCl is 4.40 and 4.28 eV, respectively. So the ECT of BiOCl is about 4.3 eV (about 288 nm) in BiOCl. With the same method, the ECT is calculated to be about 4.1 eV (about 300 nm) in BiOBr. According to the above result, we reason that broad NUV excitation band is assigned to the band gap of BiOCl rather than the CT of Eu3+-O2-. Figure S5. Emission spectra of Eu3+ (7 mol%)-doped BiOCl phosphor exciting at 358 nm with Hitachi F-7000 Fluorescence Spectrophotometer. S6 References: 1. H. Li, H. K. Yang, B. K. Moon, B. C. Choi, J. H. Jeong, K. Jang, H. S. Lee and S. S. Yi, “Crystal Structure, Electronic Structure, and Optical and Photoluminescence Properties of Eu(III) Ion-Doped Lu6Mo(W)O12,” Inorg. Chem., 50, 12522-12530 (2011). 2. X. Zhang, Z. Ai, F. Jia and L. Zhang, “Generalized One-Pot Synthesis, Characterization, and Photocatalytic Activity of Hierarchical BiOX (X= Cl, Br, I) Nanoplate Microspheres,” J. Phys. Chem. C, 112, 747-753 (2008). 3. Y. Su, L. Li and G. Li, “Synthesis and Optimum Luminescence of CaWO4-based Red Phosphors with Codoping of Eu3+ and Na+,” Chem. Mater., 20, 6060-6067 (2008). 4. L. Li, S. Zhou and S. Zhang, “Investigation on Relationship Between Charge Transfer Position and Dielectric Definition of Average Energy Gap in Eu3+-Doped Compounds,” J. Phys. Chem. C, 111, 3205-3210 (2007). 5. L. Li and S. Zhang, “Dependence of Charge Transfer Energy on Crystal Structure and Composition in Eu3+-Doped Compounds,” J. Phys. Chem. B, 110, 21438-21443 (2006). S7











