Grade 12 BIOTECHNOLOGY CURRICULUM
advertisement
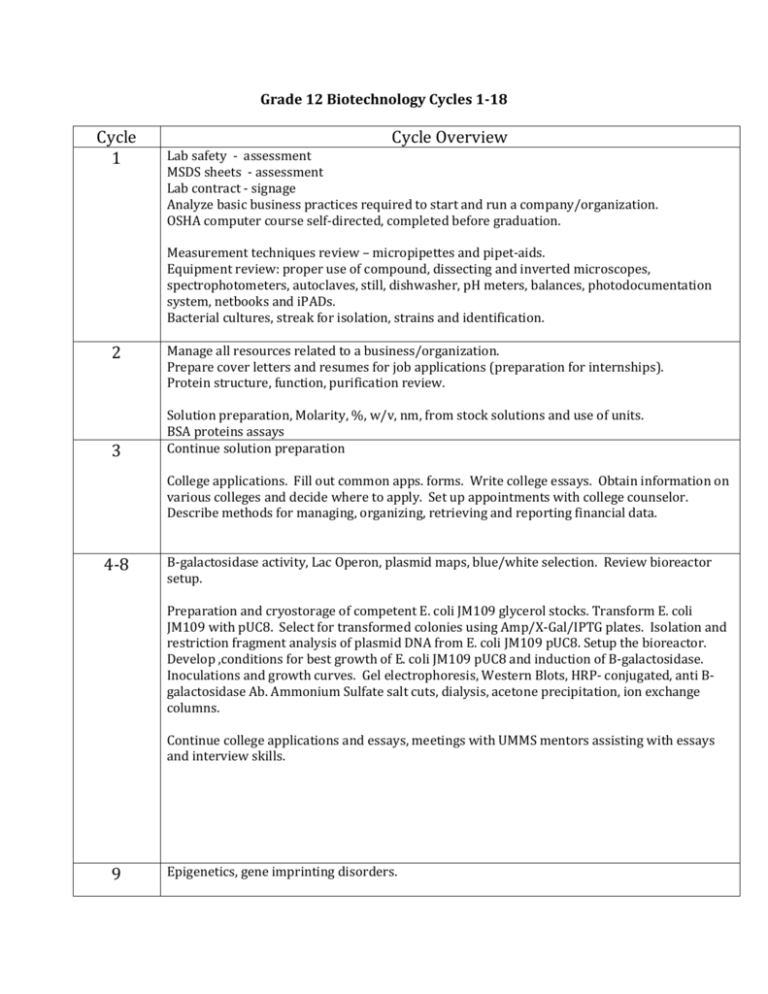
Grade 12 Biotechnology Cycles 1-18 Cycle 1 Cycle Overview Lab safety - assessment MSDS sheets - assessment Lab contract - signage Analyze basic business practices required to start and run a company/organization. OSHA computer course self-directed, completed before graduation. Measurement techniques review – micropipettes and pipet-aids. Equipment review: proper use of compound, dissecting and inverted microscopes, spectrophotometers, autoclaves, still, dishwasher, pH meters, balances, photodocumentation system, netbooks and iPADs. Bacterial cultures, streak for isolation, strains and identification. 2 3 Manage all resources related to a business/organization. Prepare cover letters and resumes for job applications (preparation for internships). Protein structure, function, purification review. Solution preparation, Molarity, %, w/v, nm, from stock solutions and use of units. BSA proteins assays Continue solution preparation College applications. Fill out common apps. forms. Write college essays. Obtain information on various colleges and decide where to apply. Set up appointments with college counselor. Describe methods for managing, organizing, retrieving and reporting financial data. 4-8 B-galactosidase activity, Lac Operon, plasmid maps, blue/white selection. Review bioreactor setup. Preparation and cryostorage of competent E. coli JM109 glycerol stocks. Transform E. coli JM109 with pUC8. Select for transformed colonies using Amp/X-Gal/IPTG plates. Isolation and restriction fragment analysis of plasmid DNA from E. coli JM109 pUC8. Setup the bioreactor. Develop ,conditions for best growth of E. coli JM109 pUC8 and induction of B-galactosidase. Inoculations and growth curves. Gel electrophoresis, Western Blots, HRP- conjugated, anti Bgalactosidase Ab. Ammonium Sulfate salt cuts, dialysis, acetone precipitation, ion exchange columns. Continue college applications and essays, meetings with UMMS mentors assisting with essays and interview skills. 9 Epigenetics, gene imprinting disorders. 11-14 Develop conditions to grow yeast in the bioreactor. Count cells using a hemocytometer, compare to cells counted using Bio-Rad counter and the Abs590 nm. Prepare growth curves. Emphasis is placed on note taking in lab notebook (or ELN). Related – During the related periods for these four weeks, frameworks 4, 5 and 6 will be reviewed as they were covered during the previous years and assessments will be taken. A final math exam will also be taken to assure the senior student goals were met. Student interns will assist with ordering, new equipment set up, inventory and freshmen instruction. Curriculum development will vary from year to year. Review protocols for amylase induction and purification Determine best conditions for induction of amylase production from pAmy in test tubes. Perform PAGE and Western blotting. Perform an amylase induction in bioreactor. Perform PAGE analysis and activity assays. Purify amylase, ammonium sulfate cuts, ion exchange, Hydrophobic interaction chromatography, PAGE analysis, activity assays 15-16 17/18 Senior interns in lab will assist freshmen with Lagomorph species identification using DNA isolation and analysis: DNA isolation, use of mitochondrial DNA for species identification, gel analysis of DNA fragment polymorphisms, PCR, optimal enzyme conditions documentation Review and take Final shop assessment. Complete all lab reports. Make up all missed work. Clean lab and store all reagents. Assist with freshmen instruction Seniors leave school.











