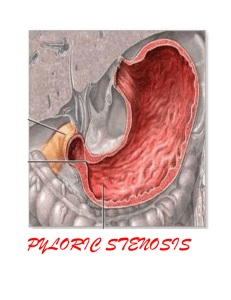
Hypertrophic pyloric stenosis (HPS) is the most common reason that
advertisement

Hypertrophic pyloric stenosis (HPS) is the most common reason that infants at about 1 month of age may require operation. The incidence ranges from 1 in 300 to 1 in 2000 live births, depending on gender and ethnicity. Boys are five times more likely to develop HPS than girls, and it occurs up to seven times more commonly in white than African-American infants.1 Five per cent of the sons and 2.5% of the daughters of fathers who have had HPS will also develop it; HPS is seen even more frequently if the mother was the affected parent, occurring in 19% of the male and 7% of the female offspring.2 The pyloric muscle in HPS becomes markedly thickened and edematous with an increase in size of the circular muscle fibers, consequently obstructing the pyloric channel. The pylorus becomes firm and discrete from the adjacent stomach and duodenum, becoming tumor-like. This pyloric hypertrophy gradually resolves following pyloromyotomy. Although extensively studied, the etiology of HPS has not been fully defined. It is felt to be multifactorial with a proven X- linked genetic factor and an unknown environmental trigger. The usual patient with HPS comes to medical attention between 3 and 6 weeks of age. The classic symptom is progressive, projectile, postprandial, nonbilious vomiting. It often starts with small amounts of emesis by the 2nd week of life, progressing to the point where all feedings are vomited. Other symptoms understandably include weight loss or failure-to-thrive and decreased frequency of bowel movements and urination. The classic finding on physical examination of an infant with HPS is a palpable pyloric mass or "olive," present in up to 90% of patients.1 Other signs include dehydration (54%), visible peristaltic waves (34%), and weight <10th percentile for age (27%). Palpation of a pyloric "olive" confirms the diagnosis of HPS in the clinical setting of emesis as described above, making diagnostic imaging studies unnecessary.3 Many physicians are not confident in their ability to feel the hypertrophic pyloric mass. I use the following method of examination for evaluation of the infant pylorus. It is helpful if the stomach is empty (eg, right after the baby has vomited). Standing to the right of the infant, I use my left hand to support and elevate the baby's head and upper torso. This maneuver allows the pylorus to drop below the liver edge and flexes the legs, helping to relax the rectus abdominis muscles. The baby may be fed a small amount of glucose water to quiet him or her and facilitate this relaxation (examination of a crying infant is futile). I sweep the tips of my right index and middle fingers down over the right rectus abdominis muscle trying to feel for the pop of the pylorus as it squirts under my fingers. The mobility of the pylorus helps differentiate it from other solid retroperitoneal structures. The classic laboratory finding of hypochloremic metabolic alkalosis is only seen in 40% to 50% of current-day infants with HPS, generally in patients with a longer duration of illness with more days of vomiting with loss of gastric contents and little net intake.4 Other abnormal lab data can include ketonuria (29%) and indirect hyperbilirubinemia (2%).1 Diagnostic imaging procedures can be obtained in those vomiting infants with (1) signs of chronic obstruction (ie, weight loss, dehydration, hypochloremic metabolic alkalosis) in whom an experienced examiner is unable to palpate a pyloric mass, (2) persistent vomiting without signs of chronic obstruction if several days have elapsed with progression of symptoms, (3) persistent vomiting in whom physical status makes abdominal examination unreliable (eg, previous abdominal surgery), or (4) bilious vomiting or other clinical situations in which HPS is not suspected.3 In experienced hands, the imaging study of choice for HPS is an abdominal sonogram because there is no exposure to radiation and barium ingestion which adds volume to an already obstructed stomach and increases the possibility of vomiting and aspiration. If findings from the sonogram are negative and the vomiting persists, an upper GI series can confirm or exclude HPS and also potentially demonstrate other problems (eg, gastroesophageal reflux, duodenal stenosis, malrotation). An upper GI series is the imaging procedure of choice for vomiting infants in whom the diagnosis of HPS is not highly suspected. Surgical treatment is the standard of care for HPS. Most patients can be operated upon within 24 hours after admission to the hospital. Upon admission, the infant is made NPO and appropriate IV fluids are started to correct any volume and electrolyte deficits and establish brisk urine output. Severe dehydration may require 24 to 48 hours of fluid resuscitation. The standard surgical procedure for HPS is a pyloromyotomy, first described by Rammstedt in 1912. It is about as perfect as an operation can be with virtually no mortality, nearly universal efficacy, and a low complication rate. A right upper quadrant transverse incision is employed. The pylorus is delivered into the wound, and it is incised longitudinally and the hypertrophic muscle fibers bluntly split, allowing the mucosa to pout through the incision, thereby relieving the obstruction. Care is taken to avoid perforation all the way through the mucosa; in experienced hands, this intraoperative complication occurs in about 4% of patients1 and results in no added morbidity if detected and repaired immediately. Every surgeon has his or her own favorite post-pyloromyotomy feeding regimen. I start low volume feedings of clear fluid 8 hours postoperatively and advance in volume and concentration until full feedings of regular baby formula are achieved in about 24 hours. With this regimen, half of the patients will vomit once or twice postoperatively, but this does not delay their feeding advancement, and half of the patients go home on the first and the remainder on the second postoperative day.5 A few surgeons have begun to perform laparoscopic pyloromyotomies for HPS. Most surgeons, including myself, have not yet gone to this approach since hospital length-of-stay has not been improved upon and return to school or work is not an issue in the HPS age group. ____________________ 1. Breaux CW Jr, Georgeson KE: Hypertrophic pyloric stenosis: A review of 216 cases from 1980 to 1984. Alabama Medicine 1986;55(3):34-37. 2. Carter CO, Evans KA: Inheritance of congenital pyloric stenosis. Journal of Medical Genetics 1969;6:233-254. 3. Breaux CW Jr, Georgeson KE, Royal SA, Curnow AJ: Changing patterns in the diagnosis of hypertrophic pyloric stenosis. Pediatrics 1988;81:213-217. 4. Breaux CW Jr, Hood JS, Georgeson KE: The significance of alkalosis and hypochloremia in hypertrophic pyloric stenosis. Journal of Pediatric Surgery 1989;24:1250-1252. 5. Georgeson KE, Corbin TJ, Griffen JW, Breaux CW Jr: An analysis of feeding regimens after pyloromyotomy for hypertrophic pyloric stenosis. Journal of Pediatric Surgery 1993;28:1478-1480. ____________________ Dr. Breaux is a pediatric surgeon and surgical critical care specialist practicing in Grand Junction, Colorado.