1.0 OBJECTIVE 1.1 To lay down the procedure for Operation

1.0 OBJECTIVE
1.1 To lay down the procedure for Operation, Cleaning and Calibration of Single
Door Autoclave, Make Lance engineering - Equipment identification No: xxxxx
2.0 SCOPE
2.1
This SOP covers the Operation, Cleaning and Calibration of Single Door
Autoclave.
3.0
RESPONSIBILITY
3.1
Microbiologist
4.0
ACCOUNTABILITY
4.1 Head - Quality Control
5.0 PROCEDURE
5.1
OPERATION OF AUTOCLAVE:
5.1.1
Check the water level before starting the autoclave. If the level is down, then add purified water upto the level mark.
5.1.2
Open the door and load the necessary items to be sterilized as required.
5.1.3
Place the autoclave indicator strips at different places in the autoclave for each cycle to indicate the proper sterilization by color differentiation of the strip.
5.1.4
Close the door using the radial locking handles clockwise till it is tight.
5.1.5
Switch on the mains to supply the power followed by the toggle switch located in the control panel to activate the heat generator.
5.1.6
Set the multi port-operating valve in slow exhaust position, allow the steam to build in the outer jacket till the pressure reaches 14-17 PSI.
5.1.7
Turn the multi port-operating valve to ‘Ster’ position.
5.1.8
When the pressure gauge reaches to 5-7 PSI then remove the condensed air by opening the drain valve for 3-5minutes and close.
5.1.9
Then the pressure gauge shows 14-17 PSI and subsequently the dial thermometer shows a rise in temperature to121°C.
5.1.10
When it stabilizes at this temperature and pressure, the timing for sterilization begins.
5.1.11
Once the temperature and pressure reaches as specified above. Carry out sterilization cycle from that time to 30 minutes
5.1.12
After the stipulated sterilization time set the multi port-operating valve in
‘Slow exhaust’, for liquids in bottles and in Fast exhaust’ for all other loads.
5.1.13
In case of vacuum drying turn the multi port-operating valve in ‘Vacuum dry Position’.
5.1.14
Once the temperature in the sterilizer drops to 60°C or the pressure drops to 0 PSI, Switch off the system, Unload the items by opening the door.
5.1.15
Enter the details like Particulars of the loaded material and sterilization time in the Single door autoclave logbook as per annexure –I.
5.2
CLEANING OF AUTOCLAVE:
5.2.1 Clean the autoclave after each cycle with purified water.
5.2.2
The inner chamber shall be cleaned with 2.5% savlon or 2.5% dettol every day at the end of each cycle.
5.2.3
Keep the autoclave in slightly open while not in use.
5.2.4
Clean the drain line by pouring hot solution of tri sodium phosphate (3.0% in water) through the drain hole in the chamber for cleaning of grease, sticky substances to avoid clogging. This should be done for every fifteen days.
5.2.5
Prepare the cleaning solutions as per current version of SOP Noxxxxx(Disinfectant preparation)
5.2.6
Enter the details of cleaning in to the autoclave cleaning record as per annexure–II.
5.3
Calibration
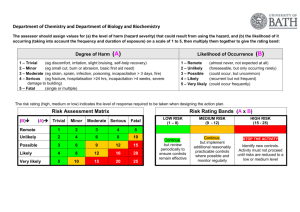
5.3.1 Validation by thermal probes
The validation of autoclave is carried out by following methods, Heat Distribution studies, Heat Penetration studies and by using biological indicator ampoules of
Bacillus sterothermophillus.
5.3.2
Heat distribution test.
5.3.2.1
Place calibrated thermal probes in different locations within the autoclave as per Appendix –I.
5.3.2.2
Place the probes in such a manner that the tip does not have any contact with the body of sterilizer.
5.3.2.3
Run the autoclave as per the point no 5.1
5.3.2.4
Record the temperature at the intervals of each minute all through the cycle.
5.3.2.5
Run the three consecutive cycles.
5.3.2.6
Place the biological indicator at or near to the probes placed.
5.3.3 Heat penetration test
5.3.2.7
Place the probes in the autoclave as per Appendix –I for minimum load and as per appendix – III for maximum load.
5.3.2.8
Place the probes in such a manner that the tip does not have any contact with the body of sterilizer.
5.3.2.9
Run the autoclave as per the point no 5.1
5.3.2.10
Record the temperature at the intervals of each minute all through the cycle.
5.3.2.11
Run the three consecutive cycles.
5.3.2.12
Place the biological indicator at or near to the probes placed.
5.3.3
Method of analysis for biological indicator
5.3.3.1
Place the ampoule / strip of Bacillus stearothermoplilus spores in a empty test tube, and number it.
5.3.3.2
Take the tubes to respective area and place them in autoclave at or near to the probes placed
5.3.3.3
After completion of sterilization cycle remove the tubes from the autoclave and carry out the analysis.
5.3.3.4
In case of strip, aseptically transfer the Bacillus stearothermoplilus strip from each tube into separate tube of sterile Soyabean casein digest medium. Mark the tubes appropriately.
5.3.3.5
Aseptically transfer a non-autoclaved Bacillus stearothermoplilus strip into a tube sterile SCDM to serve as positive control.
5.3.3.6
Mark one tube of sterile Soyabean casein digest medium as negative control.
5.3.3.7
Incubate all the tubes at 55-60°C for seven days and observe them every day for any visual evidence of growth.
5.3.3.8
In case of ampoule for Bacillus stearothermoplilus, incubate the ampoules as such at 55-60
O
C for at least 48 hours.
5.3.3.9
Keep one un-autoclaved ampoule along with the tested ampoules to serve as positive control.
5.3.3.10
Record the results in annexure –III
5.3.3.11
If there is no evidence of growth in any of the tubes except positive control or if the color of the ampoules remain purple or turns reddish brown, the results are valid.
5.3.3.12
If evidence of growth in any of the tubes except positive control tube or ampoule (color change to yellow) the results are invalid.
Repeat the test procedure using fresh Bacillus stearothermoplilus strips / ampoules.
END OF DOCUMENT