PPT here
advertisement

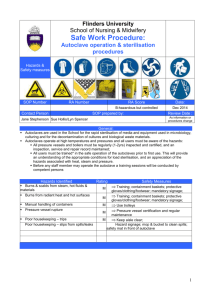
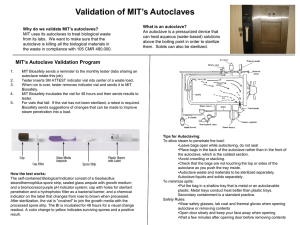
EQUIPMENT FOR AUTOCLAVING BY D.NARENDAR M. Pharm-II sem DEPARTMENT OF PHARMACEUTICS UNIVERSITY COLLEGE OF PHARMACEUTICAL SCIENCES KAKATIYA UNIVERSITY, WARANGAL CONTENTS INTRODUCTION ADVANTAGES DISADVANTAGES WHAT CAN BE AUTOCLAVED EQUIPMENT TYPYS AND OPERATION VERIFICATION VALIDATION CONCLUSION REFERENCE INTRODUCTION Sterilization: Killing or removing all forms of microbial life (including endospores) in a material or an object. Autoclaving is the process of moist heat sterilization, in which micro-organisms are exposed to steam under saturated pressure. ADVANTAGES Destroys micro-organisms more efficiently than dry heat and therefore a shorter exposure at a lower temperature is possible. Used for large proportion of official injections. Porous materials can be sterilized without damage. Equipment or components of rubber and certain plastics such as nylon and pvc. DISADVANTAGES Unsuitable for anhydrous materials such as powders and oils. Not used for injections, articles such as some plastics, that deteriorate at 115°C What can be autoclaved Surgical Instruments Glassware Plastic tubes and pipette tips Solutions and water Animal food and bedding Waste Equipment Autoclave: Chamber which is filled with hot steam under pressure. Preferred method of sterilization, unless material is damaged by heat, moisture, or high pressure. Temperature of steam reaches 121°C at twice atmospheric pressure. Most effective when organisms contact steam directly or are contained in a small volume of liquid. All organisms and endospores are killed within 15 minutes. Require more time to reach center of solid or large volumes of liquid. Principle of autoclave Steam penetrates objects in the autoclave Condensation creates negative pressure and draws in additional steam Moist heat kills microorganisms via Coagulation of proteins. Working of autoclave: Steam enters the chamber jacket, pass through an operating valve Enters the rear of the chamber behind a baffle plate It flows forward and down ward through the chamber and load, existing at the front bottom. Pressure regulator maintains pressure in the chamber and in jacket at a minimum of 15psi,the pressure required for steam to reach 121°C Conditions inside are thermostsstically controlled Types Two types of autoclaves • gravity displacement • vacuum assisted gravity : • most common • steam pumped into top of chamber • air forced out bottom during conditioning phase Pre-Vacuum Uses a vacuum pump to remove air from chamber prior to sterilization Steam able to penetrate all surfaces within the chamber Pot-vacuum drying phase ensures dryness Based on amount sterilized autoclaves are two types portable type horizontal type Autoclave cycle: Involves three phases. • conditioning • exposure • exhaust Conditioning: • first phase • steam enters chamber and conditions load • air displaced through chamber drain Autoclave cycle (contd) Exposure: Steam processes load at selected time and temperature. Effects kills of infectious agents Exhaust: Steam is removed from chamber and pressure is released Load is dried, if drying option is selected Other methods of autoclaving: Pasteurization: Developed by Louis Pasteur to prevent the spoilage of beverages. Used to reduce microbes responsible for spoilage of beer, milk, wine, juices, etc. Classic Method of Pasteurization: Milk was exposed to 65°C for 30 minutes. High Temperature Short Time Pasteurization (HTST): Used today. Milk is exposed to 72°C for 15 seconds. Ultra High Temperature Pasteurization (UHT): Milk is treated at 140°C for 3 seconds and then cooled very quickly in a vacuum chamber. Advantage: Milk can be stored at room temperature for several months. Other methods of autoclaving: Tyndallization: - heating the material at 80°C for 1hr or at 100°C for less on three successive days. - vegetative cells killing in the first heating, spores will germinate before the next, when they also will be killed. Heating with a bactericide: - heating the injection solutions in their final containers in boiling water or in a steamer at 98 to 100°C for 30 mins, the permitted bactericides being o.2% chlorocresol and o.002% phenylmercuricnitrate. - it is not used for intrathecal, intracysternal and iv injections greater than 15 ml in volume. Other Autoclaving methods: Low pressure method: Steam is admitted to a previously evacuated autoclave to give a negative pressure of about 360mm of Hg, resulting in a temperature of 80°C. It maintained for 10 mins kill all vegetative bacteria. Effectiveness of process increased by adding formaldehyde vapour to the steam and spores can be killed if conditions are held for 2 hr. VERIFICATION TESTS Monitoring of the temperature and pressure within the chamber gives indication that the process has been properly carried out. Verification of autoclaving by ٠ Instrumental method ٠ Bacteriological methods ٠ chemical indicators Instrumental method Temperature conditions within the load can be sensed by thermocouples or thermisters inserted into it various places. In case of liquids, the probe is placed in representative bottles and in case of dressings inserted well into specimen tanks. Autoclave fitted with ports and connecting to recorders which give a continuous tracing. Disadvantages: Not give information on the humidity conditions. Existence of superheating within porous loads such as surgical dressings. Chemical indicators: Previously called as witness tubes method. Consisting of a crystalline substances of known melting point contained in a glass tubes . E.g. sulphur - 115°C, acetanilide - 116°C, succinic anhydride - 120°C, benzoic acid -121°C. A dye could be included to show more clearly that the crystal had melted. Brown’s tubes method – a controlled chemical reaction is involved in the change of colour of a red liquid through amber to green. For autoclaving two types are available: Type-I -- changes to full green in about 16 min at 120C and in 10 min at 125C. Type-II -- changes in about 10 min at 120C. ● Chemical methods also available in the colour of adhesive tape(3M Ltd) or sheets of paper marked with sensitized strips(E.S.& A Robinson Ltd). These are useful when a high autoclaving temperature 134C applied with holding time of not more than 3.5 min. VALIDATION PROTOCOL Validation of the Autoclave is classified into the following 1.0 IQ _Installation Qualification 2.0 OQ – Operational Qualification 3.0 PQ – Performance Qualification VALIDATION TEAM: Installation Qualification: Name Description Model and identification no. The location Utility requirements Connections and safety features of the systems/equipment which need to be documented. OPERATIONAL QUALIFICATION PROTOCOL (OQ) 1.0 PURPOSE : To demonstrate and document that the operations of the Autoclave take place as specified . 2.0 SCOPE : Autoclave xxxxx will be qualified to meet OQ. 3.0 RESPONSIBILITY : Microbiologist, Manager Q.C 4.0 PROCEDURE : This should be performed by external agency. 4.1 Verify the following as per instrument operating procedure and calibration certificate kept in place before validation. 4.1.1 : Temperature display of Autoclave. 4.1.2 : Compound pressure gauge of Autoclave. Acceptance criteria : All calibration data found to be within the acceptable norms of calibration certificate. 4.2 Calibration of Thermocouples : Calibrate all the thermocouples of data logger before and after the validation using standard thermometer and also made available party’s calibration certificates. Acceptance criteria: The variation between the temperature of thermocouples and the standard thermometer found to be within the acceptance criteria. 4.3 Heat Distribution Studies Carry out heat distribution studies by using a multipoint data logger and maintain holding time for 15 minutes at 15 lbs. by fixing all the 12 probes as per diagram. Record the temperature and lag time of each probe. Acceptance criteria All probes must reach temperature 121-124°C and pressure must be within 15 to 18 lbs for 15min cycle. Diagram-1 Probe to 12 inside the chamber Load Pattern Maximum Load Load with all the glassware and media filled upto 70%, of the chamber and the details are as follows. Test-1 : 250ml Conical flasks = 12 Nos with media, 13 Nos without media, 500ml Conical flasks with media = 4nos, 1000ml Conical flasks with media =4nos, Pipette10ml=10nos,Pipette 2ml=10nos, Pipette 5ml=10nos, Pipette 1ml = 10nos,100ml bottles=20nos ,Filtering unit=10nos, Test tubes =25nos Minimum Load Load with all the glassware and media required for a day’s analysis (average). 250ml Conical flasks with media = 6nos, 500ml Conical flask with media – 3 nos., 1000ml Conical flask - 1 Pipette 10ml= 10nos, Pipette 2ml= 10nos, Pipette 5ml= 10nos, Pipette 1ml=10nos, Bottles=10nos, Test tubes - 25nos. Record the temperature and lag time PERFORMANCE QUALIFICATION PROTOCOL (PQ) 1.0 PURPOSE : To provide a performance qualification protocol for Autoclave. 2.0 SCOPE : Specified to Autoclave xxxxx. 3.0 RESPONSIBILITY : Microbiologist, Manager Q.C 4.0 PROCEDURE 4.1 Heat Penetration Studies: Carry out the heat penetration studies by : using a multi point data logger for the following loads mentioned. Record the temperature and lag time. Acceptance criteria: All 12 probes must reach temperature 121°C to 124°C and pressure must be within 15 to 18 lbs. for 15 min cycle. 4.2 Microbial limit test : Incubate the sterilized media flask or tubes from any one Maximum and Minimum load of heat penetration studies and observe for nutritive properties. Bacteria: 30 – 35 °C for 72 hrs, Fungi : 20 – 25°C for 120 hrs Acceptance criteria: No microbial growth should be observed i.e. Negative control and Nutritive properties of media must pass 4.3 Microbial challenge test : Keep ampoules containing spores suspension of Bacillus stearothermophilus 106 population at various location of the autoclave along with probes and maintain the sterilization temperature at 15psi and 121°C during the heat penetration studies, once on the maximum load. Acceptance criteria: Autoclaved ampoules containing Bacillus stearothermophilus spores suspension ampoules should not show any colour change after five days of incubation. 5.0 DOCUMENTATION : 5.1 Master Instrument used for validation of autoclave Institutes name and address carrying out calibration. Standard calibrating instrument name and number. Instrument certified against (Instrument of national or international standards) Date of calibration and validity period of calibration. Training certificate of persons (External agency) carrying out validation. 6.2 Autoclave being calibrated : All temperature readings for autoclave being validated should be collected from the approved external agency. Validation report with observed any error, statement of calibration and next validation due. 7.0 FREQUENCY : Once in a year until and unless no change in autoclave. In case of any change, the autoclave must be revalidated 8.0 CONCLUSION : Finally conclusion should be drawn based on the results of above tests and documented conclusion Autoclaving are used to render items (such as waste) sterile or free of any living organisms. To accomplish this, autoclaves use extreme heat, steam and pressure. Once autoclaved, waste can be disposed of in the regular trash as it is no longer hazardous. REFERENCE Ansel’s – Pharmaceutical dosage forms and drug delivery systems. Bentle’s – Text book of pharmaceutics. Cooper Gunn’s – Dispensing for pharmaceutical students. www. google. com www. Autoclaving. com www. Validation of autoclave. Com www. Moist heat sterilization. co.in www. Pharma E text book. com Lachmann – Theory and practice of industrial pharmacy Ananthnarayana – Text book of microbiology www. Wikipedia. Com James swabrick, James C. Boylan, Encyclopedia of pharmaceutical technology, Edition-3, Volume-I