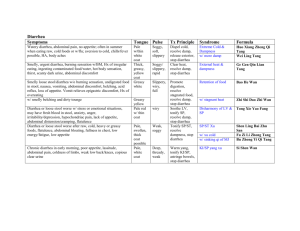
Reaction between HCl and Magnesium Reaction between HCl and
advertisement

Reaction between HCl and Magnesium Bubbles Appeared Reaction between HCl and Iron Bubbles Appeared Some bubbles appeared Nothing Happened ‘pop’ sound ‘pop’ sound Damp red litmus paper remained red. ‘pop’ sound Fire went off Damp red litmus paper remained red. Effect of gas on damp blue litmus paper Damp blue litmus paper remained blue. Damp blue litmus paper remained blue. Damp blue litmus paper turned red. Name of Gas Formed Chemical Formula of Gas Hydrogen Gas H2 Hydrogen Gas H2 Hydrogen Gas H2 ---------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------- ---------------------- Observation when metal is added to acid Effect of gas on burning(lighted)splint Effect of gas on damp red litmus paper Reaction between HCl and Zinc Damp red litmus paper remained red. Reaction between HCl and Copper Conclusion 1. What can you conclude about the reaction between some dilute acids and some metals? Dilute acids react with reactive metals to produce hydrogen gas. 2. Write the general equation for the reaction between dilute acid and metal. Metal + Acid Hydrogen + Salt 3. How can you identify the gas formed when come dilute acids react with some metals? A burning splint may be used. If a ‘ pop’ sound is heard when the splint is brought near the gas, the gas is hydrogen 4. From the effect of gas on damp litmus paper, what can you conclude about the nature of hydrogen gas? It is not reactive 5. Arrange the metals according to their reactivity in descending order. ( most reactive first) Iron, magnesium, zinc, copper