pH Scale - Magoffin County Schools
advertisement

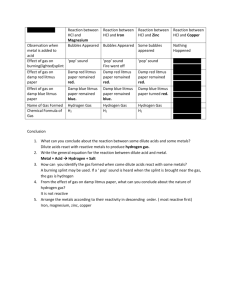
pH Scale We measure acidity and alkalinity using the pH scale, pH being short for “potential of hydrogen”. The pH scale ranges from 0 to 14. It is logarithmic, so that a difference of one pH unit represents a tenfold change in hydrogen (or rather, hydronium) ion concentration. While we are not concerned with how to calculate pH, it is important to know how pH changes with hydronium ion concentration: it is a reciprocal scale. This means that as the pH values decrease, the concentration of hydronium ion (H3O+) increases. For instance, a substance with a pH of 2 has 10 times the concentration of hydronium ion concentration as a substance with a pH of 3. As the pH values increase, concentration of hydroxide ions (OH-) increases. A substance with a pH of 10 has 100 times the hydroxide ion concentration as a substance with a pH of 8. Although it may be confusing, remember that if you hear about a solution having a low pH it actually means it is quite acidic. Water is a neutral compound, except for that very small amount-one half a billion molecules-that is dissociated. When this is translated to the pH scale, water has a pH of 7. This is considered the neutral point. Acids have pHs lower than 7, and bases have pHs higher than 7. One way to think of this is that for every pH point lower than 7, the solution has 10 times more h+ floating around than is present in regular water. Likewise, every pH point above 7 means that 10 times more OH- is present than is in water. The pH of a substance can be determined by a pH meter or by an indicator, such as litmus. Indicators turn different colors, depending on the pH of a solution. You have probably heard of a litmus test. This involves placing a drop of solution onto litmus paper, which contains the indicator, and observing the color change, if any. For instance, acids will turn blue litmus paper red, and bases will turn red litmus paper blue. However, the range over which indicators change colors is small. Litmus is only an effective indicator for solutions in the pH range form 5.5 -8.0. Therefore, chemists use many different indicators to test the pH of solutions across the entire pH scale. Again you ask, what does this mean to living things? The pH of solutions has far-reaching consequences for living organisms. For larger multicellular organisms, highly acidic solutions are often used to digest foods. One common example is stomach acid (gastric acid). It is made up mostly of HCl, a highly acidic compound. However, the pH of the stomach can range from between 1.5 to 7.0 depending on the stage of digestion. The cells lining the stomach are the only cells in the human body that can withstand strong acids. So, when foods enter the lower part of the stomach, sodium bicarbonate is added to the food slurry. The sodium bicarbonate is produced by the pancreas and released into the duodenum (lower part of the stomach). The result is a neutralization reaction where salt and water are produced. But let’s think small. What effect does pH have on the cells of organisms? Or for that matter, how does pH affect unicellular organisms? Remember pH means “potential of hydrogen”. This is just a scientific way of saying the ability of the solution to give away its protons (or hydrogen atoms, H+) a really strong acid (one with a low pH) really, really wants to give away hydrogen atoms. Conversely, a really strong base (one with a high pH) really, really wants to accept proton (or forcibly take them from other unsuspecting molecules). Adding or taking away hydrogen atoms changes the physical structure of the molecule In biological systems, the function of a molecule is highly dependent on its three-dimensional structure. Changing its structure can severely alter its function. Solutions with an extreme pH value can break apart proteins inside cells, tear down cell membranes or cell walls and, most importantly, inhibit the function of enzymes. The final result: death or destruction of the cell. Inhibiting the function of enzymes can prevent metabolic reactions form occurring. Most biological systems function best within a neutral pH range. We’ll talk more about enzymes and their importance to biological systems later in this chapter. Section Review 1. Define the following terms: a. pH scale 2. 3. 4. 5. 6. 7. b. indicator Which value represents a strong acid? a. 2 b. 7 c. 8 d. 14 Which of the following substances is basic? a. Milk b. Vinegar c. Bleach d. rainwater Orange juice has a pH of about 2 and tomato juice has a pH of about 4. Orange juice has a lower pH because a. It is more basic than tomato juice b. It is a stronger base than tomato juice c. It is a stronger acid than tomato juice d. It is a weaker acid than tomato juice Which pH value is best to use when culturing an amoeba? a. 3 b. 10 c. 14 d. 7 A solution has a pH of 12. It is a. A strong acid b. A strong base c. A neutral solution d. A weak acid How many atoms of hydrogen are in 1 molecule of glucose? (C6H12O6)? a. 3 b. 6 c. 12 d. 18