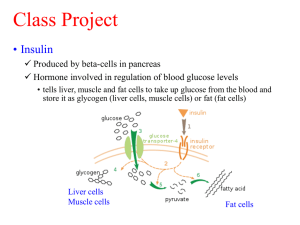
Insulin/ Gold Nanoparticle Bonding Interactions: The nature of the
advertisement

Insulin/ Gold Nanoparticle Bonding Interactions: The nature of the binding of insulin to gold nanoparticles provides an important aspect that affects the stability as well as the facility in which release of insulin from gold nanoparticles occurs. Current research has proposed nanoparticle formulations in which convalent linkages of insulin is bound to the gold nanoparticles as well as formulations through the interaction of hydrogen bonding with amino acid capped gold nanoparticles (Scheme 1). Direct covalent binding of insulin to the gold nanoparticle surface occurs through amine or thiol groups which appear on the gold nanoparticle surface. In contrast, formulations in which gold nanoparticles are capped with aspartic acid produce weaker hydrogen bonding interactions, causing facile release of insulin. Research has shown that insulin bound to bare gold nanoparticles through covalent linkages has a lower success rate (86%) in comparison to aspartic acid capped gold nanoparticles (95%). The weaker hydrogen bonding and electrostatic interactions are responsible for the faster and larger release of insulin. Reference: Langmuir 2006 article Effect of Gold Nanoparticles on Insulin Delivery-Subcutaneous Delivery Vs. Oral and intranasal delivery: In subcutaneous administration of insulin, a rapid decrease is observed in blood glucose levels from 38% to 53% after administration. In oral administration of insulin bound to gold nanoparticles as well as aspartic acid capped gold nanoparticles, a reduction in blood glucose levels of 19% and 31% respectively is observed, showing that insulin is bioactive and the aspartic acid capped gold nanoparticle surface enhances insulin uptake. However intranasal administration of insulin did not result in decrease in blood glucose levels, although when gold nanoparticles and aspartic acid gold nanoparticles were used to deliver insulin, a reduction of glucose level was observed of 50% and 55% respectively. Within intranasal delivery, the release rate of insulin produced maximum blood glucose reduction 180 minutes after administration when gold nanoparticles were used. The use of aspartic acid gold nanoparticles showed that there is a more rapid release of insulin when aspartic acid capped gold nanoparticles are used in which the maximum blood glucose reduction occurred after 120 minutes. This result also shows that the there is a greater membrane permeability of gold nanoparticle insulin formulations across mucosal cells in comparison to gastrointestinal mucous. By comparing subcutaneous administration in which a 53% reduction in blood glucose levels was observed, to aspartic capped nanogold intranasal administration, the results indicate that transmucosal delivery of insulin using gold nanoparticles is an new alternative for insulin drug delivery in comparison to painful subcutaneous delivery. Reference: Langmuir 2006 article Use/ Effects of Chitosan and Albumin The design of nanoparticles for oral and intransally delivered insulin has shown to be a new and innovative approach to the treatment of diabetes. Nanoparticles produced by formulation of ionotropic pregelation have a multilayer complex where insulin is protected within the nanoparticle with a coating consisting of proteaseprotective proteins. The nucleus of the particle consists of alginate, dextran sulfate, and insulin, which is then complexed with chitosan, and coated with albumin. Chitosan is a D-glucosamine and N-acetyl glucosamine polyamine, which is unbranched. Chitosan displays properties such as mucoadhesion, biodegradability, and biocompatible properties, which stabilize the formation of the nanoparticles., and prevent degradation The chitosan also reduce transepithelial electrical resistance, which is caused by growth of epithelial tissues and can prevent the effective delivery across the membrane. Other favorable properties of chitosan include the ability to transiently open tight junctions which are found between epithelial cells which results in enhanced insulin absorption through paracellular pathways (transfer of substances between cells of the epithelium). Chitosan acts to initiate and modulate insulin entrapment by incorporating polyanionic polymers such as dextran sulfate. These polyanionic polymers also act to increase stability, retention, and release of insulin in the gastrointestinal tract by enhancing the electrostatic interactions between the nucleus of the nanoparticle and the insulin molecules. The albumin coating is used to minimize acid degradation of the nanoparticle containing insulin which is a target for enzymatic degradation. The concentration of albumin present also has an influence on the efficiency of the entrapment of insulin. The most efficient insulin entrapment occurred (100%) when albumin concentration was between 0.25% and 0.50%. The ability to trap the insulin molecules appears to decrease as the concentration of albumin is increased which is likely due to a consequent rise in pH of the nanoparticle, reaching the isoelectric point of insulin and decreasing the electrostatic interaction between the insulin and the nanoparticle. The rate of release of insulin is also affected by the chitosen and albumin concentrations. Higher chitosan concentrations influenced the rate of insulin release by causing the nanoparticle to swell. Ions present in the gastric fluid inside the nanoparticles break the ionic interaction between the insulin and the nanoparticles. When there is a high concentration of albumin present, there is a higher insulin release due to weakening of the electrostatic interaction between insulin.