Biology
advertisement

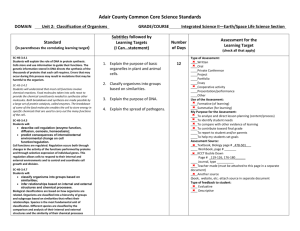
Name:______________________________ Pd:_____Date:_______________________ WATER AND ITS ROLE IN BIOLOGY Water is in LIQUID form at most temperatures on the Earth’s Surface. Water expands when it freezes, enabling it to float. Water covers ~75% of the Earth’s surface. Most abundant compound in nearly all organisms Greatest SOLVENT in the world Known as the Universal Solvent Uneven distribution of electrons makes it POLAR : slightly positive at one end and slightly negative at the other Because of its POLARITY it has strong attractive forces other polar molecules Cohesive Force- attraction of molecules of the same substance, why some insects can “walk on water” Adhesive Force- attraction of molecules of different substances, this is the force that causes water to rise up in a narrow tube against the force of gravity. This effect is called capillary action. Example: water moving from the ground intoa plants roots and up to the stems and leaves. CHART OF BIOLOGICAL IMPORTANCE OF WATER: PROPERTY OF WATER Ice is less dense than water SIGNIFICANCE FOR LIVING ORGANISMS Ice floats and also insulates the underlying water high surface tension Water forms droplets on surfaces and runs off Low viscosity Liquid at room temperature Water flows through very small spaces and capillaries Liquid medium for aquatic life and inside cells Colourless with a high transmission of visible light Light penetrates tissue and aquatic environments Strong cohesive properties and high tensile strength Many substances can dissolve in water (known as the universal solvent) Water can be lifted and does not pull apart easily Significant amounts of energy are required before water will change its state Medium for the chemical reactions of life (metabolism) water is the main transport medium In organisms Contents of cells are unlikely to freeze In order for water to evaporate it must absorb a large amount of energy Water can absorb a lot of energy for only a small rise in temperature Heat is lost by evaporation of water. Sweating and transpiration causes rapid cooling Aquatic environments are thermally stable. Organisms have stable internal temperatures when the external temperature is fluctuating. Questions: 1. In the space below, draw a water molecule indicating its polarity. 2. With respect to aquatic ecosystems, explain the likely biological consequences if ice was DENSER than liquid water: 3. For each example, state the property of water and explain its biological importance a. Clarity of seawater b. Water travelling up xylem tissue in plants c. Transport of glucose around the human body 4. Create a concept map on the properties of water. Include the following terms: hydrogen bonds, polarity, cohesion, adhesion, capillary action, solvent, solute, hydrophobic, and hydrophilic Name:______________________________ Pd:_____Date:_______________________ WATER AND ITS ROLE IN BIOLOGY Water is in ___________ form at most temperatures on the Earth’s Surface. Water ________________ when it freezes, enabling it to ______________. Water covers _________ of the Earth’s surface. Most abundant __________________ in nearly all organisms Greatest SOLVENT in the world Known as the ___________________________________ Uneven distribution of electrons makes it _________________: slightly positive at one end and slightly negative at the other Because of its POLARITY it has strong attractive forces other __________________________ ______________- attraction of molecules of the same substance, why some insects can “walk on water” ________________________- attraction of molecules of different substances, this is the force that causes water to rise up in a narrow tube against the force of gravity. This effect is called capillary action. Example: water moving from the ground intoa plants roots and up to the stems and leaves. CHART OF BIOLOGICAL IMPORTANCE OF WATER: PROPERTY OF WATER Ice is less dense than water high surface tension Low viscosity Liquid at room temperature Colourless with a high transmission of visible light Strong cohesive properties and high tensile strength Many substances can dissolve in water (known as the universal solvent) Significant amounts of energy are required before water will change its state SIGNIFICANCE FOR LIVING ORGANISMS In order for water to evaporate it must absorb a large amount of energy Water can absorb a lot of energy for only a small rise in temperature Questions: 5. In the space below, draw a water molecule indicating its polarity. 6. With respect to aquatic ecosystems, explain the likely biological consequences if ice was DENSER than liquid water: 7. For each example, state the property of water and explain its biological importance d. Clarity of seawater e. Water travelling up xylem tissue in plants f. Transport of glucose around the human body 8. Create a concept map on the properties of water. Include the following terms: hydrogen bonds, polarity, cohesion, adhesion, capillary action, solvent, solute, hydrophobic, and hydrophilic