Overview of Statistical Reporting
advertisement

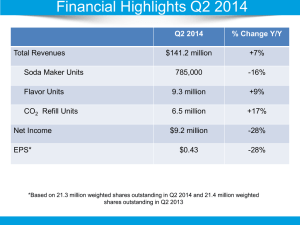
#HHSN268201100025C INTERMACS Interagency Registry for Mechanically Assisted Circulatory Support INTERMACS Operating Instructions Title: Overview of Statistical Reporting: Public/Complete Document Number: IOI-10-101-V1 Document Storage Location: M:\INTERMACS 2011-2015\10 Statistical Reports\2 IOI Public\IOI-10-101-V1 Overview Of Statistical Reporting.Docx Author: Position: Ryan Cantor Statistical Reports Director Approved by: Position: Ryan Cantor Statistical Reports Director Approved Date: Effective Date: Last Edited Date: 07/19/2013 07/19/2013 07/19/2013 Page 1 of 5 IOI-10-101-V1 Overview of Statistical Reporting Overview of Statistical Reporting 1. PURPOSE AND SCOPE The purpose of the DCC Statistical Reports Department is to provide descriptive summaries of patients, devices, and outcomes on a routine basis to a variety of stakeholders. Statistical Reporting from the DCC reaches a wide audience. There are several registries (INTERMACS, pediMACS and MEDAMACS) and several audiences (Federal Partners, Industry, and Sites) including other internal departments. Major efforts have been taken to streamline the statistical reporting processes to run with parallel processes across registries and across reports. These techniques encourage efficiency, accuracy, and ease of process maintenance. 2. PERSONNEL / RESPONSIBILITIES The key roles and tasks for the Statistical Reports Department are: Statistical Reports Director – manages the creation and distribution of all routine DCC statistical reports. Tasks include the oversight of all statistical programming used in the process, training of staff, documentation, and interfacing with other departments to ensure the most accurate and timely reports are generated Report Distribution Manager – organizes tasks involved with report validation and distribution and directly communicates with sites regarding reporting questions Programming Assistant / Report Support – supports the generation of reports through a variety of tasks including process maintenance, documentation, and SAS job submission Programming Assistant / Data Management Support – supports both the Data Management Department and Statistical Reports Department in maintaining routine data processing duties Medical Device Reporting (MDR) Manager and Support Staff – MDR processing and submission duties to the FDA and industry Page 2 of 5 IOI-10-101-V1 Overview of Statistical Reporting 3. PROCEDURE The following tables list the INTERMACS Statistical Reports INTERNAL REPORTS Report Registry Compliance Report Industry Billing Report Distribution Quarterly Quarterly Audience Nurses for Audits Finance INDIVIDUAL REPORTS Report Federal Partners Report CMS Report Industry Report – Thoratec Industry Report – SynCardia Industry Report – HeartWare NHLBI Report Distribution Quarterly Quarterly Quarterly Quarterly Quarterly Annually Audience Federal Partner, OSMB, Public CMS Thoratec SynCardia HeartWare NHLBI SITE REPORTS Report Site Reports Quality Assurance (QA) Data Quality (DQ) Distribution Quarterly Audience Sites The following tables list the pediMACS Statistical Reports INDIVIDUAL REPORTS Report Federal Partners Report Distribution Quarterly Audience Federal Partner, OSMB, Public SITE REPORTS Report Site Reports Distribution Quarterly Audience Sites Page 3 of 5 IOI-10-101-V1 Overview of Statistical Reporting A general outline of the steps used to generate and distribute reports is provided in the figure bellow. Page 4 of 5 IOI-10-101-V1 Overview of Statistical Reporting 4. SCHEDULE Reports are generated on a quarterly basis. After the end of a reports coverage date, an additional month of data is collected to allow site coordinators to enter all forms that apply to the report. The Data Management Department receives raw data and processes the analytical datasets used in the reports. Reports are drafted and edits and new exhibits are implemented. Finally in the last week of the quarter the reports are distributed. Quarterly Report Schedule: Calendar Quarter Report Coverage Stop Date Dataset* Report Distribution Deadline Q1 Q2 Q3 Q4 March 31st June 30th September 30th December 31th April July October January June 30th September 30th December 31th March 31th * Reports are generated from datasets one month after coverage stop date, to allow time for data entry. 5. SUPPORTING DOCUMENTATION AND RESOURCES Complete step by steps instructions for all DCC Statistical Reporting processes can be found in the internal document IOI-10-001 Statistical Reporting. Revision 01 Author Ryan Cantor Revisions Made New IOI Date 07/019/2013 Page 5 of 5