Glucose-6-phosphatase dehydrogenase deficiency (Favism) Abstract

2014/12/27 1 st
edition
Glucose-6-phosphatase dehydrogenase deficiency (Favism)
Abstract
Glucose-6-phosphatase dehydrogenase deficiency (G6PD deficiency or favism) is the most common enzyme deficiency in humans. It is inherited as an X-recessive linked disorder. It has a high prevalence in persons of African, Asian and Mediterranean descent. G6PD deficiency can present as neonatal hyperbilirubinemia. People with this disorder can experience episodes of brisk hemolysis in response to oxidative stresses or, less commonly, have chronic hemolysis. However, many individuals with
G6PD deficiency are asymptomatic.
Etiology and epidemiology
The G6PD enzyme is part of the pentose monophosphate shunt. It catalyzes the oxidation of glucose-6-phosphate and the reduction of nicotinamide adenine dinucleotide phosphate (NADP+) to nicotinamide adenine dinucleotide phosphate (NADPH). NADPH maintains glutathione in its reduced form, which acts as a scavenger for dangerous oxidative metabolites.
Red blood cells depend on G6PD activity to generate NADPH for protection, which is THE ONLY way to generate NADPH. Therefore, red blood cells are more susceptible to oxidative stresses than other cells. In person with G6PD deficiency, oxidative stresses can denature hemoglobin and cause intravascular hemolysis.
The World Health Organization has classified the variants into class
I-V according to the degree of enzyme deficiency and severity of hemolysis. The degree of G6PD deficiency determines the clinical expression of the disorder, from with no symptom to encountering episodes of brisk hemolysis triggered by infection, drugs, fava beans, or ketoacidosis.
Class Enzyme activity
I Severe (< 10%)
Description
With chronic nonspherocytic hemolytic anemia
II Severe (< 10%) With intermittent hemolysis
III Moderate (10~60%) Hemolysis with stressors only
IV Normal (60~150%) No clinical sequelae
V Increased No clinical sequelae
G6PD deficiency occurs worldwide. G6PD deficiency confers partial protection against malaria. Thus, the geographic prevalence of the disorder correlates with the distribution of malaria. The highest prevalence rates
(with gene frequencies from 5-25%) are found in the following regions:
1.
Tropical Africa
2.
The Middle East
3.
Tropical and subtropical Asia
4.
Some areas of the Mediterranean
5.
Papua New Guinea
Risk factors
A.
Antimalarial drugs: Primaquine, pamaquine and chloroquine.
B.
Sulfonamides: Sulfanilamide, sulfamethoxazole and mafenide
C.
Drugs for mycobacterium: Thiazolesulfone, dapsone, isoniazid
D.
Other antibiotics: Nalidixic acid, nitrofurantoin and furazolidone
E.
Analgesics: Aspirin, phenazopyridine and acetanilide
F.
Substances: Methylene blue, naphthalene and Henna
Signs and symptoms
Most persons with G6PD deficiency are asymptomatic. Symptomatic patients can present with neonatal jaundice and acute hemolytic anemia.
Neonatal jaundice usually appears within 24 hours after birth, simutaneous or slightly earlier than physiologic jaundice. In rare situation, neonatal jaundice will affect central nervous system, which is called kernicterus. This complication may cause dysfunction of motor or sensory, mental retardation and even fatal result
Acute hemolytic anemia can occur due to oxidant stress induced by exposure to certain drugs, chemicals, substances (Camphor, fava beans and so on) and disease status (Infections or ketoacidosis). It begins 24~72 hours after exposure to oxidant stress. Clinical manifestations include weakness, tachycardia, jaundice, and hematuria. The disease course is about 8~14 days and then subsides spontaneously due to production of young red blood cells with high levels of G6PD.
Patients with severe G6PD deficiency have chronic hemolysis and are often thought to have non-spherocytic hemolytic anemia.
Diagnosis
Test
CBC
BCS
Urinalysis Hemoglobulin(+), hemosiderin(+)
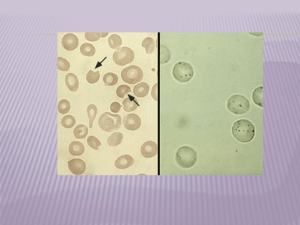
Blood smear Polychromasia, Heinz bodies
Result
Rbc ↓ , Hb ↓ , % Reticulocyte ↑
LDH ↑ , T-/I-bil ↑ , haptoglobin ↓
Treatments
Most patients do not need treatment. They should be taught to avoid factors that can cause oxidant stress. Identification and discontinuation of the precipitating agent is critical to manage hemolysis in patients with
G6PD deficiency. Anemia should be treated with appropriate measures,
Infants with prolonged neonatal jaundice as a result of G6PD deficiency should receive phototherapy. Exchange transfusion may be necessary in cases of severe neonatal jaundice or hemolytic anemia.
Patients with chronic hemolysis or non-spherocytic anemia should be placed on daily folic acid supplements. Consultations with a hematologist and a geneticist should be sought.
Edited by NDU medical office
Resource: G6PD deficiency, Wikipedia http://en.wikipedia.org
G6PD deficiency, Medscape http://emedicine.medscape.com/article/200390-overview