A-Biochemistry Unit
advertisement

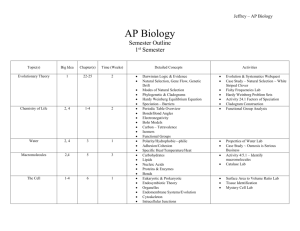
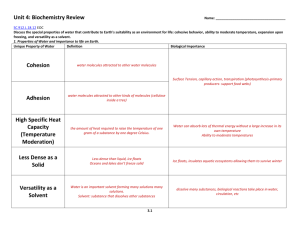
Unit Title: Biochemistry Grade: 10th grade Biology Biochemistry Enduring Understandings: Matter is made up of minute particles called atoms, which are composed of even smaller components (protons, neutrons, & electrons) that have measurable properties, such as mass and electrical charge Elements are arranged and grouped in the periodic table based on their properties. The periodic table is used to predict common properties of elements Atoms interact with each other by transferring or sharing valence electrons forming ionic or covalent bonds. Outer electrons govern the chemical properties Carbon forms the backbone of bio-compounds because of its ability to form bonds with a wide variety of element Chemical energy is potential energy stored in chemical bonds. Chemical reactions can absorb energy or release energy. Chemical reactions can absorb energy or release energy. Enzymes are special biological catalysts (proteins) that lower a reaction’s activation energy to speed up the chemical reaction in living systems. They are affected by temperature, and pH. Water has unique properties that makes life possible on earth. For example, may appear in a solution and works as a solvent to make mixtures and compounds. Acids and bases play a role in keeping an organism’s internal pH at an acceptable range Life elements consist of Carbon, Hydrogen, Oxygen, Nitrogen, Phosphorus, and trace elements to help form key nutrients There are four important organic compounds known as macromolecules that are responsible for life, carbohydrates, proteins, lipids and nucleic acids. Cells link monomers together to form polymers to build macromolecules and store energy and break off monomers to release energy or break down macromolecules into subunits depending on cell needs. Carbohydrates are an important energy source for cell processes such as saccharides, starches, and glucose Lipids provide energy, protective coverings, membrane components, and protective padding such as triglycerides and phospholipids Proteins are important to life processes in building and repair, controlling biochemical reactions, and genetic instructions. The process of science helps biologists investigate how nature works at all levels, from the molecules in cells to the biosphere. Nucleic acids carry genetic information for reproduction and protein synthesis such as Amino Acids, DNA and RNA. 1 Unit Title: Biochemistry Grade: 10th grade Biology Essential Questions: How does life depend on atoms and their interactions? What does the position of an element on the Periodic table tell us? What is the matter in organisms made of? How do chemicals combine and break apart inside of living things? How do organisms use different types of carbon compounds? How are lipids, carbohydrates, proteins and nucleic acids important to living things? How do the processes of ‘dehydration synthesis’ (condensation) and ‘hydrolysis’ contribute to the building and breaking of macromolecules? How are enzymes important to metabolic processes and homeostasis? Why is the cycling of matter important to life on Earth? What is the difference between and acid and a base? Why is the analogy of the “lock-and-key” and “induced fit” models of used to model enzyme activity? What are some factors that affect enzyme activity? The process of science helps biologists investigate how nature works at all levels, from the molecules in cells to the biosphere. 2 Unit Title: Biochemistry Grade: 10th grade Biology Biochemistry Critical Skills: Critical Content: Carbohydrate Lipid Protein Nucleic Acid Macromolecules Biogeochemical (Matter) Cycle Nucleotide Nucleus Chemical Bonds Enzyme Water Phospholipids Triglycerides pH/acid/base Buffer Nitrogen Nutrient Denitrification Nitrogen Fixation Carbon Oxygen Hydrogen Dehydration Synthesis Hydrolysis Saturated Unsaturated Ionic Bond Element Atom Covalent Bond Nitrogen fixation Organic compound Inorganic compound 3 Identify the components of macromolecules Combine element symbols to form chemical formulas to explain biochemical reactions Assemble the periodic table to demonstrate the location of each nutrient element and its characteristic based upon its properties and characteristics. For example, N, Cl-, K, Na, H, O, C, S, Fe, I, Ca+, etc. Explain the biogeochemical cycles and their contributions to maintain a delicate balance between life and the environment. Model of dehydration-synthesis and hydrolysis reactions in biochemical processes. Compare and contrast the importance of ‘dehydration synthesis’ (condensation) and ‘hydrolysis’ in macromolecule bonds. Unit Title: Biochemistry Grade: 10th grade Biology Critical Content: Critical Skills: Inorganic compound Molecule Amino acid Polypeptide Bond Phosphorous Sulfur Periodic table Particle Matter Electron Energy Adenosine Triphosphate Adenosine Diphosphate Saccharide Monosaccaride Polysaccharide Hydrogen Bond Starch Chemical Reaction Deoxyribose Nucleic Acid (DNA) Ribonucleic Acid (RNA) Adhesion Cohesion van der Waals forces Ion Activation energy Isotope Solution Solute Mixture Suspension Solvent Monomer 4 Examine the “lock-and-key” and “induced fit” models of enzyme activity Outline the significance of the four macromolecules and their functions essential to all living organisms. Explain of important types of biochemical reactions. Identify the components of the ADP and ATP cycle. Explain the energy storage and release in the ADP ATP reaction in cells. Evaluate the properties of enzymes and the optimal conditions needed for enzyme reactions. Demonstrate the unique properties of water and its relevance to life processes. Analyze the Atomic theory and structure of the atom. Unit Title: Biochemistry Grade: 10th grade Biology Big Idea: All living organisms are made of atoms and arise from the building blocks (macromolecules) of water (the universal solvent) and carbon CHO. Water and the macromolecules for life: protein, carbohydrates, lipids, and nucleic acids are essential to life. Equally important are the physical bonds and particles of matter that determine the behavior of a molecule. Learning Targets 3.4 The Interdependence of Organisms 3.4.1 The atoms and molecules on the earth cycle among the living and nonliving components of the biosphere. 3.5 Matter, Energy, and Organization in Living Systems 3.5.1 All matter tends toward more disorganized states. 3.5.2 The energy for life primarily derives from the sun. 3.5.3 The chemical bonds of food molecules contain energy. 3.5.4 The complexity and organization of organisms accommodates the need for obtaining, transforming, transporting, releasing, and eliminating the matter and energy used to sustain the organism. 3.5.5 The distribution and abundance of organisms and populations in ecosystems are limited by the availability of matter and energy and the ability of the ecosystem to recycle materials. 3.5.6 As matter and energy flows through the different levels of organization of living systems--cells, organs, organisms, communities--and between living systems and the physical environment, chemical elements are recombined in different ways. 5 Focused Assessed Unit Title: Biochemistry Grade: 10th grade Biology Performance Tasks and Suggested Learning Experiences with Ideas for Differentiation R)=Remediation E)=Extension Construct/demonstrate a molecular model in 3D demonstrating the bonding properties between two different monomers to form a polymer. Sell a compound in a brochure format highlighting the compounds characteristics and behaviors. Students will take an oral or written exam of differentiated assessment practices: true and false, multiple choice, essay, matching, short answer, and completion. Students will generate a media video project to present to the class on the 4 macromolecules. Why is the process of building and breaking molecules important to life?” See “My Cool Video” Appendix 1 Student build a model of a macromolecule A paper-scissors-tape activity used to help students envision the process of synthesis -- using water to build macromolecules out of smaller subunits see: http://www.explorebiology.com/apbiology/labs/ Students will fill in a template of the Periodic Table using templates and tiles demonstrating the system and order of the families/groups and periods, etc. Identification of critical life elements and the process of the biogeochemical cycles in the form of a cartoon or storybook: 6 Unit Title: Biochemistry Grade: 10th grade Biology Carbon/Oxygen cycle = Carbohydrate and lipids Nitrogen Cycle = DNA/RNA/Nucleic Acids/Amino Acids/Proteins Phosphorous Cycle = Adenosine Triphosphate (cell energy) Water Cycle = Building and Breaking of Molecules Students build diagrams that explain the ATP and ADP cycle to identify the forming and breaking of bonds. Explanation of the energy storage and release in the ADP ATP reaction in cells utilizing enzymes. Explanation of how enzymes work on a lock and key model. Have students explain and sketch the process to a partner or small group and then have them repeat it again to another student. Essay: The unique properties (characteristics) of water make life possible on Earth. Select three properties of water and: a. for each property, identify and define the property and explain it in terms of the physical/chemical nature of water b. for each property, describe one example of how the property affects the functioning of living organisms. Identification of important types of biochemical reactions. Identification of the properties of Enzymes and the optimal conditions needed for enzyme reactions. Students will create a poster that depicts the important details of one cycle and present in 2 min. its contribution to the cycle of life. Make sure that multiple students will be presenting on the same cycle and all cycles are assigned. Have small groups of students create concept maps relating the concepts: atom, proton, neutron, electron, element, isotope, compound, ionic bond, ion, covalent bond, and molecule. Afterwards have each group share with the class and allow groups to make adjustments as needed. Labs: Bonding Lab (Ionic vs covalent) using bags of popcorn kernels and papers marked Na or Cl Are foods acidic or basic Hydrolysis Demonstration Give students one minute to write a short paragraph explaining why one (carbon, water, phosphorous, nitrogen) cycle is important for sustaining 7 Unit Title: Biochemistry Grade: 10th grade Biology life on Earth. Bromothymol blue indicator lab (CO2 + H2O H2CO3 demonstrates that carbonic acid is a weak acid Household items or a foods acidic or basic lab Properties of water: Water Cohesion and Adhesion Lab Hydrolysis Demonstration Crime Lab Macromolecules Macromolecules Lab Enzyme Toothpick Activity: http://www.explorebiology.com/documents/Lab10Toothpickase2005.pdf 8 Unit Title: Biochemistry Grade: 10th grade Biology Resources Biology, by Miller and Levine (Pearson) The Science Teacher’s Book of Lists, by Barhydt and Morgan http://www.explorebiology.com/apbiology/labs/ “My Cool Science Video Project” © COPYRIGHT 2001-2010 Joan Vandervelde http://www.microbeworld.org/ www.middleschoolscience.com www.sciencespot.com 9