Exam 1 SP2015

CHM 2020 SP2015 Wilson
Name ________________________________________________ Date ______________
Part 1: Organic Chemistry I Review
1.
Identify the functional groups by matching the given structures with the provided options. Write the letter in the box located under each structure. (9 points)
A. Alcohol
B. Alkene
C. Aldehyde
D. Alkyne
E. Amine
F. Amide
G. Carboxylic acid
H. Epoxide
I. Ester
J. Ether
K. Ketone
L. Nitrile
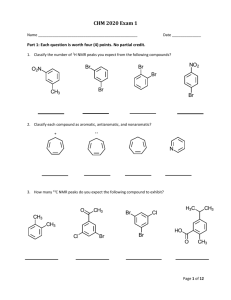
O
NH
2
H
O
O
O
OH H
3
C C N
O
O
OH
Page 1 of 12
CHM 2020 SP2015 Wilson
2.
Identify the relationship between the following pairs of molecules as configurational isomers, conformational isomers, or constitutional isomers. Please circle one of the answer choices provided below each pair of molecules. (2 points)
OH
O configurational isomer conformational isomer constitutional isomer configurational isomer conformational isomer constitutional isomer configurational isomer conformational isomer constitutional isomer configurational isomer conformational isomer constitutional isomer
3.
Draw the enantiomer and two diastereomers of the following molecule with the proper wedge and dash notation in the allocated spaces. (3 points)
OH
NH
2 enantiomer diastereomer diastereomer Page 2 of 12
CHM 2020 SP2015 Wilson
4.
Label the stereogenic centers in each molecule as R and S using the allocated spaces. (5 points)
O
NH
Cl
OH
NH
2
H O
O
OH
O
O
N
H
N
O
O
OH
5.
Draw nine constitutional isomers for C
7
H
16
. (9 points)
Page 3 of 12
CHM 2020 SP2015 Wilson
Part 2: Radical Reactions
6.
Categorize the following radical intermediates in order of decreasing stability with one (1) being the most stable and four (4) being the least stable. (1 point)
.
.
.
.
7.
Predict the major product(s) of the following reaction and indicate stereochemistry. (6 points)
H
3
C Br h
+ Br
2
CH
3
8.
Propose an efficient synthesis to accomplish the following reaction transformation. Provide reagents and any intermediate compounds. (8 points)
Br
Br
Page 4 of 12
CHM 2020 SP2015 Wilson
9.
Draw the corresponding monomer or polymer. (4 points)
Polymer
Ph Ph Ph
Monomer
COOEt
10.
An unknown compound has a chemical formula of C
9
H
20
produces three constitutional isomers upon chlorination but only one isomer is isolated upon radical bromination. Circle the isomer that is the most possible structure of the unknown molecule. (1 point)
I
I
II
II
III
III
IV
IV
V
V
VI
VI
Page 5 of 12
CHM 2020 SP2015 Wilson
Part 3: Conjugation, Resonance, and Dienes
11.
Identify the following compounds as having cumulated, conjugated, or isolated double bonds.
(1 point)
O
C
12.
Draw the major product formed from the following reactions. (4 points)
+
1 equivalent
HBr
+
0 °C
1 equivalent
HBr
13.
Provide two different pathways to prepare the following compound by the Diels-Alder reaction and explain why one pathway is preferred. (4 points)
O
O
Page 6 of 12
CHM 2020 SP2015 Wilson
14.
Provide the diene and dienophile used to produce the following Diels-Alder products. (8 points)
O
O
O
CHO
CHO
O
O
O
15.
Circle the molecule that will absorb light at a longer wavelength. (1 point)
O
Page 7 of 12
CHM 2020 SP2015 Wilson
Part 4: Benzene and Aromatic Compounds
16.
Identify the following molecules as aromatic, antiaromatic, or not aromatic. (2 points)
S
O
O
N
O
N
H
O
O
O
C
-
N
17.
Consider the following two molecules. How would you distinguish between them using 1 H and 13 C
NMR spectroscopy? (4 points)
Page 8 of 12
CHM 2020 SP2015 Wilson
18.
Provide a common or systemic name for each of the following molecules. (4 points)
O
CHO
OH
Cl
OH
Br
O
2
N
Cl
19.
Predict the major product of the following reactions. (2 points)
NO
2
NO
2
Na
2
CrO
7
NBS
H
2
SO
4
, H
2
O
20.
Circle the molecule would you expect to be most acidic. Justify your choice. (2 point)
O
Page 9 of 12
CHM 2020 SP2015 Wilson
Part 5: Aromatic Substitution Reactions
21.
Predict the major product(s) of the following reactions. (5 points)
H
2
SO
4
+
OH
H
3
CO
CCl
3
OCH
3
+ SO
3
H
2
SO
4
+
HNO
3
H
2
SO
4
N
H
O
O
+ Br
2
FeBr
3
O
2
N
O
+
Cl
AlCl
3
Page 10 of 12
CHM 2020 SP2015 Wilson
22.
Provide the structure of the major intermediate product(s) in the following reaction sequence.
(6 points)
Cl
2
FeCl
3
Cl
AlCl
3
Br
2
h
OCH
3
Ph
O
Cl
AlCl
3
HNO
3
H
2
SO
4
23.
Predict the product(s) for the following reaction . (4 point)
CH
3
NaNH
2
NH
3
Cl
Cl
NO
2
NO
2
O NH
Fe
HCl
Page 11 of 12
CHM 2020 SP2015 Wilson
24.
Write the complete reaction mechanism for the following reaction and predict the product.
(5 points)
AlCl
3
Cl
?
Page 12 of 12