CHM 2020 Exam 3 SP2014
advertisement

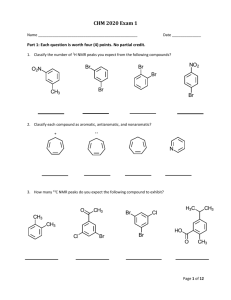
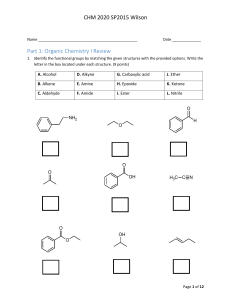
CHM 2020 Exam 3 Name _________________________________________ Date ___________________ Part I. Functional group review. Each question is worth two (2) points each. There is no partial credit. (12 points) 1. Match the given structures to appropriate functional group represented by writing the matching letter in the space provided under each structure. O O NH2 O N H HN O C N Cl A. Acid chloride B. Acetal C. Aldehyde D. Alcohol E. Amide F. Amine G. Anhydride H. Enamine I. Ester J. Imine K. Ketone L. Lactam M. Lactone N. Nitrile Page 1 of 11 CHM 2020 Exam 3 Part II. Multi-step synthesis reactions. Each reaction transformation is worth two (2) points each. There is no partial credit. (18 pts) 2. Use the space provided below to write the chemical formula or draw the structure of the reagent(s) used to complete each reaction transformation. NO 2 A + NH2 N N B Cl A C Br - B C 3. Use the space provided below to write the chemical formula or draw the structure of the reagent(s) used to complete each reaction transformation. A Br N3 B NH2 C NH O A B C 4. Use the space provided below to write the chemical formula or draw the structure of the reagent(s) used to complete each reaction transformation. O A NH2 NH2 C B N N H A B C Page 2 of 11 CHM 2020 Exam 3 Part III. Reaction Review. Each question is worth three (3) points each. There is no partial credit. (27 points) 5. Draw the major organic product(s) formed in the following reaction transformations. Show stereochemistry if applicable. 1. KOH 2. 1-bromohexane O NH 3. NaOH, H2O O O O O O + O NaOEt O EtOH 1. Br2, AcOH 2. pyridine O 1. excess Br2, KOH 2. H2O Page 3 of 11 CHM 2020 Exam 3 NH2 1. NaNO2, HCl 2. H2O O O O 1. NaOEt O O 2. H2O, HCl 1. LDA, -78 °C 2. CH3CH2CH2Br O O NaOEt EtOH NH2 1. Excess CH3I 2. Ag2O, H2O 3. heat Page 4 of 11 CHM 2020 Exam 3 Part IV. Synthesis. Each question is worth five (5) points each. (10 points) 6. Determine the product, E, of the following reaction sequence. Draw the structure of intermediates A – D to receive partial credit. Please label each intermediate with the appropriate letter. (10 points) O 1. NaBH4 H A SOCl2 NaCN B C 1. LDA, -78 °C D 2. CH3CH2Br 2. H2O 1. LiAlH4 E 2. H2O Cl Product E 7. Determine the product, D, or the following reaction sequence. Draw the structures of intermediates A – C to receive partial credit. Please label each intermediate with the appropriate letter. (8 points) O 1. NaOEt O 2. O A 1. NaOEt 2. CH3CH2Br B H3O+, heat C 1. LDA D 2. Br O 3. H3O+ Product D Page 5 of 11 CHM 2020 Exam 3 Part V. Spectroscopy. 8. Match the correct constitutional isomer with the appropriate 1H NMR spectra below. Write the letter of the spectra A, B, C, or D in the box located below each compound. (12 points) H N NH2 N N H Spectra A Spectra B Page 6 of 11 CHM 2020 Exam 3 Spectra C Spectra D Page 7 of 11 CHM 2020 Exam 3 Part VI. Reactions Conditions Match-up. Each question is worth two (2) points each. There is no partial credit. (8 points) 9. Determine the correct reaction conditions to produce B from the starting material, A. Place an X over the incorrect reaction conditions. O O O LDA, -78 °C NaOEt, 25 °C Br B B A 10. Draw the expected product from the selected incorrect reaction conditions. 11. Determine the correct reaction conditions to produce C from the starting material, A. Place an X over the incorrect base and reaction conditions. O O O LDA, -78 °C NaOEt, 25 °C Br C A C 12. Draw the expected product using the selected incorrect reaction conditions. Page 8 of 11 CHM 2020 Exam 3 Part VII. Concept Review. Each question is worth two (2.5) points each. There is no partial credit. (5 points) 13. Circle the thermodynamic enolate of 2-methylcyclohexanone? O - O - O - O - 14. Circle the carbonyl compounds that do not undergo self- aldol reactions with treated with NaOH in H2O? O O O H H O O O H Page 9 of 11 CHM 2020 Exam 3 Part VII. Retrosynthesis. Each question is worth four (4) points each. (8 points) 15. Draw the reactants used to produce the following compound from a crossed Claisen reaction. O O KOH H2O 16. Draw the reactants needed to prepare the following compound using a Robinson Annulation. KOH O H2O Page 10 of 11 CHM 2020 Exam 3 Page 11 of 11