CHM 2020 Exam 2 SP2014
advertisement

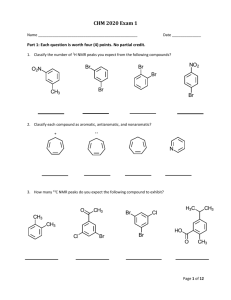
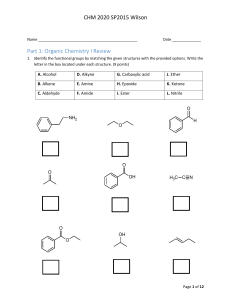
CHM 2020 Exam 2 Name _________________________________________ Date ___________________ Part I. Functional group review. Each question is worth two (2) points each. There is no partial credit. (12 pts) 1. Match the given structures to appropriate functional group represented by writing the matching letter in the space provided under each structure. O HN O O O NH O O O A. Acid chloride B. Acetal C. Aldehyde D. Alcohol E. Amide F. Amine G. Anhydride N H. Enamine I. Ester J. Imine K. Ketone L. Lactam M. Lactone N. Nitrile Page 1 of 12 CHM 2020 Exam 2 Part II. Multi-step synthesis reactions. Each reaction transformation is worth two (2) points each. There is no partial credit. (18 pts) 2. Use the space provided below to write the chemical formula or draw the structure of the reagent(s) used to complete each reaction transformation. O O A OH N O Cl B O C O N NH2 N A N B C 3. Use the space provided below to write the chemical formula or draw the structure of the reagent(s) used to complete each reaction transformation. O A Br O B OH C NH2 NH2 A B C 4. Use the space provided below to write the chemical formula or draw the structure of the reagent(s) used to complete each reaction transformation. O A A O OH B O Br B C C Page 2 of 12 CHM 2020 Exam 2 Part III. Reaction Review. Each question is worth two (2) points each. There is no partial credit. (18 pts) 5. Draw the major product(s) formed in the following reaction transformations. O 1. H3O+, SH SH 2. Raney Ni O O excess H2 O O Pt O 1. NaBH4 O 2. H2O O CrO3 H H2SO4, H2O Page 3 of 12 CHM 2020 Exam 2 O 1. (CH3CH2CH2)2CuLi 2. H2O 1. O3 2. CH3SCH3 or DMS O CH3NH, DCC OH O HO H2SO4 OH 1. CH3CH2CH2Li N 2. H2O Page 4 of 12 CHM 2020 Exam 2 Part IV. Mechanism. 6. Draw a stepwise mechanism for the following reaction. (14 points) O O O cat. TsOH, H2O + HO OH Page 5 of 12 CHM 2020 Exam 2 Part V. Spectroscopy and Synthesis. 7. Draw product, E, of the following reaction sequence. Use the corresponding 1H NMR spectrum to confirm if the product is ortho or para substituted. Draw and label products A thru E to receive partial credit (10 points) O Br CH3COCl A AlCl3 OH HO B H3O+ Mg C H D H3O+ PCC E Product F Page 6 of 12 F CHM 2020 Exam 2 Part VI. Spectroscopy. No partial credit. 8. Match the correct constitutional isomer with the appropriate 1H NMR spectra below. Write the name of the spectra A, B, C, or D in the box located below each compound. (12 points) O H O H O H O H Spectra A Spectra B Page 7 of 12 CHM 2020 Exam 2 Spectra C Spectra D Page 8 of 12 CHM 2020 Exam 2 9. Use the following 1H and 13C NMR spectra to determine the correct constitutional amide. Please circle your answer. (6 points) O N O O N H2N H Page 9 of 12 CHM 2020 Exam 2 Part VI. Reagent Review. Please circle one reagent from the options given to answer the questions provided below. Each question is worth 2 points. No partial credit. (10 points) 10. Which reagent should you use to convert an aldehyde or ketone into an alkene? Gilman reagent Tollens reagent Wittig reagent 11. Which reagent should you use to reduce an ester to an aldehyde? DIBALH LiAlH4 NaBH4 12. Which reagent should you use to convert an amide into a nitrile? H3O+ LiAlH4 SOCl2 13. Which reagent should you use to reduce the alkene only in an α, β – unsaturated compound? (1 equiv.) H2, Pt NaBH4 Excess H2, Pt 14. Which reagent is used to oxidize an aldehyde to a carboxylic acid? KMnO4 Ag2O, NH4OH PCC Page 10 of 12 CHM 2020 Exam 2 Page 11 of 12 CHM 2020 Exam 2 Page 12 of 12