Worksheet on Ionic and Covalent/Molecular Compounds Answers
advertisement

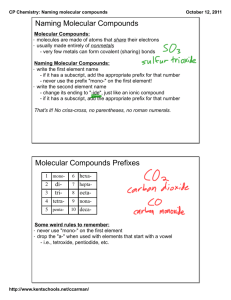
Ionic Compounds II and Molecular Compounds Answers Name:__________________________ Period:____________ Part A: Ionic Compounds II 1. How many moles of atomic entities are there in 1.5 mol. of each following compound? a. Iron(II) bicarbonate b. Barium phosphate c. Cobalt(III) sulfite d. Aluminum dichromate e. Cesium oxalate f. Copper(II) nitride 2. For each of the compound in Q1, calculate molar mass. a. Iron(II) bicarbonate b. Barium phosphate c. Cobalt(III) sulfite d. Aluminum dichromate e. Cesium oxalate f. Copper(II) nitride 3. For each of the compound in Q1, calculate mass for each atomic entity in 10.00 grams of corresponding compound. a. Iron(II) bicarbonate b. Barium phosphate c. Cobalt(III) sulfite d. Aluminum dichromate e. Cesium oxalate f. Copper(II) nitride Part B: Binary Molecular Compounds Binary molecular compounds are composed of two types of atoms connected by covalent bonds. The following are the conventions used in naming binary molecular compounds: a) Select the element with lower electronegativity, put it first and use the element name. b) The element with higher electronegativity is put in the 2 nd place and its name changed to end with -ide. [So far, quite similar to the binary ionic compounds. But the problem is that there are no charges associated with molecular compounds since there is no complete transfer of valence electrons. Hence, number of atoms associated with each compound has to be stated clearly.] c) Use prefixes (see table below) to indicate how many atoms are present in each molecule for molecular compounds. Only exception is mono- prefix for the first element is omitted. Number 1 2 3 4 5 6 7 8 9 10 Prefix monoditritetrapentahexaheptaoctanonadeca- Examples: NO: nitrogen monoxide [mononitrogen monoxide, the mono- prefix for nitrogen(the first element) is dropped. However, the mono- prefix for the 2nd element has to be kept.] S2F4: disulfur tetraflouride Write out the name for the following compounds. 1. CO ___________________ 2. CO2_________________________________ 3. N2O___________________ 4. N2O4_______________________________ 5. NO2_________________________________ 6. CF4__________________________________ 7. BCl3_________________________________ 8. PCl5_________________________________ 9. SF6__________________________________ 10. S2O3_________________________________ Write out the formulas for the following. 1. phosphorus trichloride ________ 2. dinitrogen pentoxide__________ 3. nitrogen triiodide ____________ 4. sulfur tetrabromide __________ 5. selenium hexachloride ________ 6. nitrogen monoxide __________ 7. sulfur dioxide ______________ 8. boron trifluoride ___________ 9. carbon tetrachloride _________ 10. arsenic pentabromide _________ Extension Question: How could one tell the difference between ionic and molecular compounds? Are there any exception(s) to the rule?