Solubility Curves
advertisement

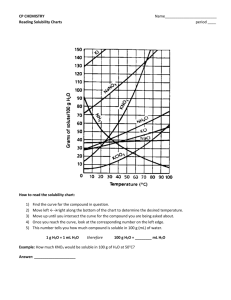
Solubility Curves- Interpreting Graph SCH3U Name: Use the table below to answer the following questions. Solubility of Some Substances in Water @ Various Temperatures Solubility (g/100 g of H20) Substance Formula 0°C 20°C 50°C Ammonium chloride Barium hydroxide Barium sulfate Calcium hydroxide Lead (II) chloride Lithium carbonate Potassium chlorate Potassium chloride Sodium chloride Sodium nitrate Sodium sulfate Silver nitrate Lithium bromide Cane Sugar (sucrose) NH4Cl Ba(OH)2 BaSO4 Ca(OH)2 PbCl2 Li2CO3 KClO3 KCl NaCl NaNO3 Na2SO4 AgNO3 LiBr C12H22O11 29.4 1.67 0.00019 0.189 0.6 1.5 4 27.6 35.7 74 4.76 122 143 179 37.2 31.89 0.00025 0.173 0.99 1.3 7.4 34 36 88 62 222 166 230.9 50.4 100°C 77.3 0.00034 0.07 1.7 1.1 19.3 42.6 37 114 50 455 203 260.4 0.7 56 57.6 39.2 182 41 733 266 487 1. Saturated solutions of each of the following compounds are made at 20°C. Circle the letter(s) of the solution(s), which will form a precipitate upon heating. a) NaCl b) Na2SO4 c) Li2CO3 d) Sucrose 2. A saturated solution of potassium chloride is prepared in 100.0 g of water at 20°C. If the solution is heated to 50°C, how much more KCl must be added to obtain a saturated solution? [8.6 g KCl] 3. A saturated solution of sucrose in 1000.0 g of boiling water is cooled to 20°C. What mass of rock candy will be formed? [2.561 Kg] 4. Using the data from the table, plot the solubility curves of KCl, LiBr, NaNO3 and Na2SO4 on the graph below. Be sure to label each curve. Use the graph to answer the following questions. 350 Solubility (g/100g of water) 300 250 200 150 100 50 10 20 30 40 50 60 70 80 90 Temperature ˚C W1 SCH3U Solubility Curves- Interpreting Graph Name: USING SOLUBILITY CURVES ANSWER THE FOLLOWING QUESTIONS: 1. If 50 grams of water saturated with ammonium chloride at 40C is slowly evaporated to dryness, how many grams of the dry salt will be recovered? [23g] 2. What is the smallest mass of water required to completely dissolve 23 grams of ammonium chloride at 40C? [50g] 3. A saturated solution of NaNO3 in 100 grams of water at 40C is heated to 50C. What is the rate of increase in solubility in grams per degree?[0.8 g/0C] 4. Which salt has solubility values that are least affected by temperature? [NaCl] 5. If 30 grams of KCl is dissolved in 100 grams of water at 45C, how many additional grams of KCl would be needed to make the solution saturated at 80C? [20g] 6. At what temperature do potassium chlorate and potassium nitrate have the same solubility in water? [50 0C] 7. At 25C, 100 grams of water is saturated with NaClO3. How many grams of NaClO3 will fall out of solution when it cools to 0C? [30g] 8. At 50C, 100 grams of water is saturated with NaCl. How many grams of NaCl will precipitate when the solution is cooled to 40C? [0.5g] 9. How many grams of sodium chloride are required to saturate 500 grams of water at 100C? [196g] 10. Which compound is least soluble in water at 12C? [KClO3 ] 11. Which compound is most soluble in water at 50C? [NH4NO3] 12. At 80C, 100 grams of water is saturated with KCl. How many grams of KCl will precipitate when the solution is cooled to 45C? [12g] 13. A saturated solution of which compound contains 132 grams of solute per 100 grams of water at 70C. [NaNO3, KNO3] 14. How many grams of sodium nitrate, are required to saturate 200 grams of water at 10C? [162g] 15. Which saturated solution of a chloride has the greatest percentage by mass of solute at 60C? [NH4Cl] W2 SCH3U Solubility Curves- Interpreting Graph Name: W3