Synthesis/Decomp Stoichiometry Notes
advertisement

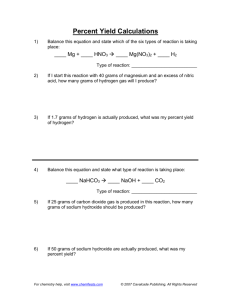
Synthesis and Decomposition Stoichiometry What is STOICHIOMETRY? It is a way of calculating (theoretically) how much product you are going to make if you know how much of each reactant you have; OR, it is (theoretically) how much of each reactant you will need to make a specific amount of product. To solve a stoichiometry problem, all you need is a balanced equation and the map above. Work through the example problem below: The decomposition of baking soda (sodium bicarbonate) uses heat and results in solid sodium carbonate, carbon dioxide gas, and water vapor as products. Balanced Formula Equation: Given 42.0 grams of baking soda, how many grams of each product are produced by the decomposition of sodium bicarbonate? Theoretical grams of sodium carbonate: 42.0 g NaHCO3 Theoretical grams of carbon dioxide: 42.0 g NaHCO3 Theoretical grams of water: 42.0 g NaHCO3 On Your Own: Liters of carbon dioxide:? 1. The synthesis reaction of hydrogen gas with chlorine gas produces hydrogen chloride gas. How many grams of hydrogen and chlorine gas are needed to produce 146.4 grams of hydrogen chloride gas? 2. What volumes of the gaseous reactants in liters, at STP, are needed? 3. The decomposition of mercury (II) chloride produces liquid mercury and a gaseous product. How many grams of mercury (II) chloride are needed to produce 100.3 grams of liquid mercury?