THURSDAY 11/6/2014 STANDARD UNIT MNES8.C.3.1.1 Describe
advertisement

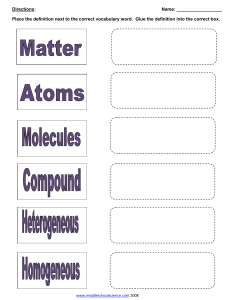
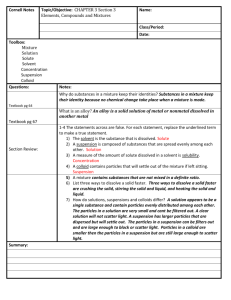
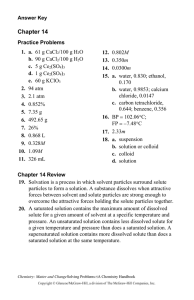
THURSDAY 11/6/2014 STANDARD UNIT MNES8.C.3.1.1 Describe forces acting on objects (e.g., friction, gravity, balanced versus unbalanced). S8.C.3.1.2 Distinguish between kinetic and potential energy. S8.C.3.1.3 Explain that mechanical advantage helps to do work (physics) by either changing a force or changing the direction of the applied force (e.g.,simple machines, hydraulic systems). YOU DO Copy HW Using Chem4kids link supplied Using Computers Take Quiz on Liquids and record using Cornell Method in Notebook. Also complete the definitions and 3 Relate tasks assigned http://www.chem4kids.com/ click matter-starting at solids- read and define http://www.chem4kids.com/files/matter_solid.html Define: electrons, concentration, crystal lattice, allotrophs, Relate: Mohs Hardness Scale to diamond vs graphite Relate: Tetrahedron and hexagon to carbon Relate: cohesive and gravity forces to affect on liquids page 94 15 C 16 D 17 A page 95 18 solution-when the solute complete dissolves so it is bonded to the solvent gas solutions Air made of nitrogen, oxygen, carbon dioxide, and other trace gases solid solutions Alloys like brass or steel liquid solutions tea suspension-when a solution has solute suspended in the solvent example snow globe colloid a mixture of two phases of matter where the solute particles are very small and do not settle out in any reasonable amount of time, cloudy is a good clue to colloid example milk or jello 19 The particle size stays the same for each atom that makes up the solution, but the space between the atoms increases The number of particles remains the same The speed of the particles increases page 96 20 Chemical changes create new substances by breaking bonds of the reactants and forming new bonds in the products, but the same amount of matter and/or energy is equal. Physical changes do not create new substances. Chemical Changes FIVE-FLAMABILITY CATS-COLOR CHANGE OPENED-ODOR FIVE-FIZZ OR FOAM PRETTY-PRECIPITATE RED-REACTIVITY WITH WATER ENVELOPES-ENERGY CHANGE Physical Changes Saturday-solubility My-malleability ductile Child’s -conductivity Wombat-weight mass Died-Density Making me-magnetism Sad-state of matter Three signs chemical changes have taken place are color change, odor, or fizzing. An increase in temperature will speed up a chemical reaction. 21 D=m/V D=38.6g/2cm3 D=19.3 g/cm3 Gold Color Weight Magnetism does not attract to a magnet so it is not iron or containing iron.