File - Ashley Fuentes`s ePortfolio
advertisement

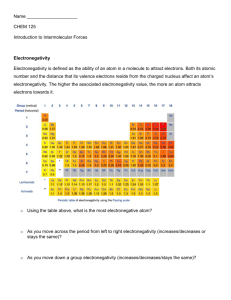
Name : Ashley Fuentes Molecule Polarity Lab Introduction: In this atomic-level simulation, you will investigate how atoms' electronegativity value affects the bonds they produce. When two atoms bond, a pair of electrons is shared between atoms. Electronegativity is a measure of a single atom's ability to attract the electrons shared in that bond. In this lab you will work to answer a number of questions. 1. From what you've already learned about the protons and electrons in an atom, what would cause an atom to have a high electronegativity value? An atom would have high electronegativity because; it has a lot of valence electrons up to 8. More than 4 and less than 8 valence electrons. 2. Why might an atom have a low electronegativity value? An atom might have a low electronegativity value by the atom being bigger. If the atom is bigger, it will have more electrons shielding its positive nucleus. Part I: Procedure Turn on (check) all view options. Take your time and investigate how the binary compound's bond behaves when the atom's electronegativity and orientation are changed. Do not rush through this step. 3. Describe the bond formed between two atoms with similar, low electronegativities. The bond formed between the two atoms is similar when at the same “less” electronegativity level. The two atoms maintain to be more covalent when at the same level. The bond dipole tends to become smaller. 4. Describe the bond formed between two atoms with similar, high electronegativities. Investigation 3.1: Electronegativity and Polarity Page 1 The bond formed between the two atoms is similar when at the same “high” electronegativity level. The bond dipole still becomes smaller. & they still end up to be more covalent. 5. Describe the bond formed between two atoms with very different electronegativities. The bond between the two atoms with “very different” electronegativity levels will make the bond dipole larger. They stand to become more ionic. 6. Describe what is meant by partial charges, δ- and δ+. a. δ- represents: negative particles 7. δ+ represents: positive particles 8. What happens when the electric field is applied to a very polar molecule? The atoms end up moving in a specific direction and paste. a. Why do you think this is? Because the atoms electronegativity levels are different. 9. Select the surface view that shows “Electron Density”. How does the density around a partial positive (δ+) compare to the density of a partial negative (δ-)? A positive partial haves less density when a negative partial haves more density. 10. Please describe what you think electron density is, in your own words. To me electron density is particles, and atoms that have to deal with or contain density. Investigation 3.1: Electronegativity and Polarity Page 2 11. What would cause the electron density to increase around an atom? The electronegativity level. A bond is characterized as ionic or covalent by comparing the differences between two atoms' electronegativities 12. Give an example of an ionic compound. a. Chemical Formula: NaCl b. Name: Sodium Chloride c. Describe this ionic compound in terms of each atoms' electronegativity values. The sodium would have a less electronegativity value and the chloride would have a higher value. 13. Give an example of a molecular covalent compound. a. Chemical Formula: SO2 b. Name: sulfur dioxide c. Lewis Dot Structure: O S O d. Molecular Geometry: 3 atoms – Linear ( double bond ) e. Describe this molecule in terms of each atoms' electronegativity values. The sodium and dioxide will end up becoming a higher electronegativity value Investigation 3.1: Electronegativity and Polarity Page 3 Name : Ashley Fuentes Part II: Procedure Additionally, we further separate covalent bonds into polar covalent and nonpolar covalent. 14. What would cause a bond to be polar covalent? uneven sharing of electrons. 15. What would cause a bond to be nonpolar covalent? Even sharing of electrons. In this simulation realize that in addition to changing the electonegativities, you may also move individual atoms by dragging them with the mouse. Here, in addition to bond polarity (represented by the bond dipole), the entire molecule may be polar (represented by the molecular dipole). The molecular dipole determines the overall polarity of the molecule and how it interacts with other molecules and its environment. BTW: The molecular dipole is found by adding the bond dipoles together while taking into account the direction of the bond dipole; think a tug-of-war. Take some time and adjust each of the atom's locations and electronegativity values several times. Observe how the bond dipoles (between A-B and B-C) add or subtract to produce the overall molecular dipole. 16. Draw an example of a molecule you created that has two strong bond dipoles but has no molecular dipole at all. A B C a. What could this molecule be? H2O Investigation 3.1: Electronegativity and Polarity Page 4 17. How can a molecule have no molecular dipole? A molecule can have no molecular dipole if the bond dipoles cancel each other out by pointing in exactly opposite directions. 18. Draw an example of a molecule you created that has two strong bond dipoles but has no molecular dipole at all. O C O Bond dipole Bond dipole a. What could this molecule be? Carbon dioxide Investigation 3.1: Electronegativity and Polarity Page 5






![QUIZ 2: Week of 09.03.12 Name: [7pts] 1.) Thoughtful list of 3](http://s3.studylib.net/store/data/006619037_1-3340fd6e4f1f4575c6d8cf5f79f0ff3e-300x300.png)
