Enzyme Catalysis with Catalase
advertisement

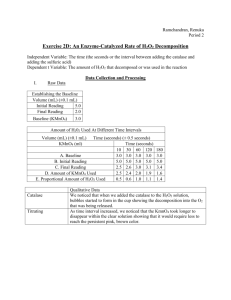
Enzyme Catalysis with Catalase Key Notes From Lab Handout • • • • Enzyme: Catalase (from beef liver) Substrate: H2O2 (hydrogen peroxide) Stop Reaction: use sulfuric acid (H2SO4) Quantify Amt. of H2O2 left: titrate in KMnO4 (potassium permanganate) • Baseline: Starting total amount of H2O2 • FOUR ways enzyme activity can be altered: – – – – Salt concentration (salinity) pH (acid/base concentration) Temperature Activators & Inhibitors Lab #3- Enzyme Catalysis w/Catalase Three Parts to the lab: • Establish Baseline Amount of H2O2 • Uncatalyzed Decomposition of H2O2 • Time Trials w/Catalase to determine Rxn rate • Procedure: – – – – – – – 10 ml H2O2 in a beaker 1.0 ml (H2O or Catalase) 10 ml 1 M H2SO4 Mix well Take a 5 ml sample and titrate in KMnO4 Read Initial and final measurements on buret Record Data Uncatalyzed data • Final reading: 11.2 ml • Initial reading: 8.1 ml • Amount of KMnO4: 3.1ml Sample Data Set I Amt. KMnO4(ml) 10 sec 30 sec 60 sec 90 sec. 120 sec. 180 sec. 360 sec Baseline 4.4 4.4 4.4 4.4 4.4 4.4 4.4 Final reading 12.0 15.3 17.4 23.1 24.4 11.5 11.6 Initial Reading 7.8 12.0 15.3 21.3 23.1 10.6 11.5 Amount KMnO4 4.2 3.3 2.1 1.8 1.3 0.9 0.1 0.2 1.1 2.3 2.6 3.1 3.5 4.3 used Amount of H2O2 used Sample Data II Amt. KMnO4(ml) 10 sec 30 sec 60 sec 90 sec. 120 sec. 180 sec. 360 sec Baseline 3.4 3.4 3.4 3.4 3.4 3.4 3.4 Final reading 8.6 10.2 11.2 12.2 11.5 11.6 11.6 Initial Reading 6.1 8.6 10.2 11.6 11.2 11.5 11.6 Amount KMnO4 used 2.5 1.6 1.0 0.6 0.3 0.1 0.05 (one drop) Amount of H2O2 used 0.9 1.8 2.4 2.8 3.1 3.30 3.35 Conclusion Questions to Consider 1) Determine the amount of H2O2 in moles using the chemical equation on pg. 22 and your amount of H2O2 after 180 sec. 2) From Question #6, Choose two factors that can affect the rate of enzyme-catalyzed reaction of Catalase. Draw a predicted graph for each reaction. 3) Name two organisms that could be used to determine the reaction rate of an enzyme. 4) List two major sources of error and one suggestion on how to improve the lab. What would you do differently?