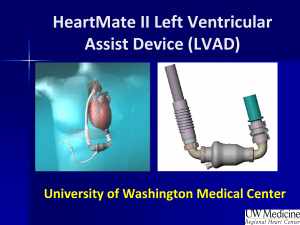
The Heartmate II LVAD
advertisement

Session C1 Bioengineering 2333 THE IMPROVEMENT OF AN UNHEALTHY HEARTH WITH THE HEARTMATE II LVAD Patrick Devlin (pjd40@pitt.edu), Patrick Bianconi (pab99@pitt.edu) Abstract—This paper will describe the significance of Thoratec’s Heartmate II Left Ventricular Assist Device (LVAD) and other ventricular assist devices (VAD) of the like, and how these devices are improving the lives of the many sufferers of heart failure. This paper will also explain how the Heartmate II compensates for an injured hearts’ inability to pump blood at an efficient rate. The wide range of eligible patients and potential costs will be assessed. The benefits of this device and existing clinical outcomes will also be discussed with great detail. Such benefits include durability of the device, longevity of the device, and success of the device [10]. This paper aims to educate the reader about heart failure, and how the innovative design and the efficient processes of the Heartmate II can sustain the life of a patient with this condition. First, the significance of heart failure and the methods of improving inept heart function will be discussed. Second, the mechanical design and the reliability of the Heartmate II will be described. Lastly, the results of clinical trials and the ethical issues surrounding artificial organs and machines will be debated. All in all the Heartmate II is affecting the lives of thousands and will continue to improve the lives of millions. through the aorta. If one of the ventricles fails to operate correctly, a ventricular assist device would be a sufficient solution to the overbearing problem [1]. Clinical research studies have showed Thoratec’s Heartmate II Left Ventricular Assist Device to be safe and effective, with improved quality of life and survival compared with historical norms for heart-failure patients [2]. The Heartmate II is not the only ventricular assist device on the market, but comparisons shall be made between this device and devices of the like, including total artificial hearts. The advantages and disadvantages of these devices will be discussed in detail, in order to explain why most attention should be paid to Thoratec’s Heartmate II Left Ventricular Assist Device. HEALING AN UNHEALTHY HEART Currently about six million patients in the United States are suffering from heart failure with an additional six hundred thousand cases arising annually [1]. About fifty percent of these sufferers die within only five years of the initial diagnosis [3]. After the initial diagnosis, about a quarter of the patients proceed to advanced heart failure. Advanced heart failure consists of the final two stages of the four stages of heart failure. The first two stages involve little or no limitation to physical activity. During stage three the patient is considered to have advanced heart failure and experiences fatigue from normal physical activity. Unlike the previous three stages, the patient experiences cardiac insufficiency while at rest during the fourth stage and has trouble doing any sort of physical activity. Less than thirty percent of patients who are suffering from advanced heart failure fail to survive more than a year. One may think that a simple solution to this crisis would be the transplantation of a heart from a donor. Unfortunately, there are less than three thousand donor organs available world-wide per year [1]. These organs could potentially save, if not severely help the lives of those suffering with any stage of heart failure. In 1964, research and development of artificial heart technologies had gone underway and continues to progress in a positive direction [4]. This information alone should be evidence enough as to why further research and development of implementing artificial heart technologies should be continued, in hopes of healing the many unhealthy hearts, not only in America, but around the world. Key Words—total artificial heart, Heartmate II, heart failure, left ventricular assist device (LVAD), transplant, THE SIGNIFICANCE OF HEART FAILURE Every year, hundreds of thousands of individuals are diagnosed with heart failure, and on top of that, millions are already suffering from this condition [1]. Heart transplants may be the best option for a healthy future, but unfortunately there are a very limited number of donor organs available and for most patients, only specific areas of the heart are in need of medical attention [1]. One may think that total artificial hearts hold the key to a healthy life for many, but this is not necessarily true. Total artificial hearts are much less common than ventricular assist devices due to the fact that not all patients qualify for one, or need a totally new heart for that matter. The heart is a muscular organ that pumps blood to all parts of the body. Oxygen rich blood from the lungs enters the heart and fills the left atrium. As blood fills up the left atrium, the mitral valve is pushed open and blood flows in the left ventricle. As the heart beats, the ventricles contract, and the blood is forced into the aorta. The aorta is the largest artery in the body, and it has the power to supply the whole body with oxygenated blood. If problems arise in the left ventricle, insufficient blood will be supplied to the body Artificial Heart Technologies Before Thoratec’s Heartmate II LVAD hit the market, other left ventricular assist devices and total artificial hearts were University of Pittsburgh Swanson School of Engineering Twelfth Annual Freshman Conference April 14, 2012 1 Patrick Devlin Patrick Bianconi the main focus of artificial heart technologies [4]. Both VADs and TAHs have the potential to be used as long term heart support or as bridges to heart transplantation. Different devices work better for the specific purpose they are serving. The main goal of all of these devices is to support a human life with sufficient blood flow throughout the body. Without this sufficient flow of blood, multiple organ failure occurs [4]. The obvious difference between VADs and TAHs is the severity of implantation. Although both procedures are invasive, the implantation of a TAH is much more dangerous, and involves a complex procedure of completely withdrawing the patient’s heart in an attempt to insert a totally artificial machine. One major drawback of TAHs is that most of devices on the market are intended for inpatient use only [4]. This severely limits the patient’s mobility and social life. Along with residing in the hospital, the patient has to wait until a heart is available for transplantation. Very few of the devices offer an option for destination therapy. A total artificial heart is a complex machine and is made up of multiple moving parts. Due to the complexity, many problems have the potential to occur [4]. This requires severe surveillance of the patient, such as hemodynamic management and anticoagulation. Routine care is also conducted and includes supporting and recovering end-organ function, respiratory and ventilator management, infection control, maintenance or nutrition, pain control, and gradual mobilization [4]. A VAD is a much simpler device in comparison and the surgery is much less invasive. The patient does not lose his or her heart, but rather has this miniscule machine assist the ventricular flow in an ordinary manner. Ventricular assist devices represent a method of providing temporary support for those patients not expected to survive until a heart becomes available for their transplant [5]. Due to the scarcity of donor hearts available, improvements have been made, allowing patients to use an assist device in a hospital setting or even as an outpatient. These improved devices are surgically placed entirely within the thoracic and abdominal cavity and are connected to the power source by a drive line that passes through the torso with an open wound. This poses obvious problems such as infection and the risk of damaging the power line. Devices like these are intended for long-term use (at least a year), but devices specifically for short term use are available for patients who are unable to use a device for the long term. Many VADs are used as bridge to transplant, but the Heartmate II in particular is FDA approved for both bridge to transplant and destination therapy. Patient management is required after implantation, but this management is not nearly as extensive as the management required after the implantation of a TAH [6]. Food and Drug Administration for transplant-ineligible heart-failure patients [7]. This device was originally approved for “bridge to transplant” in 2008, but on January 20th, 2010, the device was approved for destination therapy for patients with end-stage heart failure who are not suitable for cardiac transplant [7]. This allows for a course of treatment for a patient who has no option of being provided with a heart for transplantation. Now less sick sufferers of heart failure can significantly increase their quality of life with the Heartmate II without using the device as a bridge to transplantation. Aside from being available to suit two different needs of patients with heart failure, the Heartmate II is very reliable and can fit a wide variety of patients in need. The current model is smaller than previously approved LVADs, meaning that it can be implanted in women and even children. The device covers the full output of a heart by pumping up to 10 liters of blood per minute and is much easier to implant than previous LVAD devices [8]. The Hearmate II is implanted alongside a patient’s heart and takes over the pumping ability of the weakened heart. Unlike total artificial hearts and even previous LVADs for that matter, the Heartmate II is made up of only one moving part and operates more simply [8]. This allows for the device to be used much longer and operate much more quietly than previous devices. A study was conducted to determine whether results with the Heartmate II LVAD in a commercial setting are comparable to other available devices for the same indication [9]. Many characteristics were similar in the results of the Heartmate II versus the other devices, except that creatine and blood urea nitrogen levels were lower in the patients with the Heartmate II. The percentage of patients reaching transplant, cardiac recovery, or ongoing LVAD support by six months was 91% for the Heartmate II and 80% for the control group [10]. At one year, survival for the Heartmate II was at 85% versus the 70% of the control group [10]. This information is strong evidence as to how the Hearmate II is very reliable in comparison to other similar devices. These data suggest that after approval, the technology has been associated with continued excellent results. The simple complexity of this device separates the Heartmate II from all other LVADs on the market. For being the first LVAD approved for destination therapy and as a bridge to transplantation, the Heartmate II can brighten the future of the many sufferers of advanced heart failure. How It Works The Heartmate II is about the size of a D-cell battery, weighs 375 grams and incorporates an electromagnetic DC brushless motor to provide rotation to the dual cup-socket ruby bearing supported impeller which has demonstrated a five year service life. The pump is made of titanium and the knitted polyester graft is reinforced with a titanium ring and a rubber sleeve [8]. THE HEARTMATE II LVAD Thoratec’s Heartmate II LVAD is the first-continuous flow left ventricular assist device approved by the United States 2 Patrick Devlin Patrick Bianconi The Heartmate II operates with a continuous flow rotary pump. Continuous flow pumps have the distinct advantages of small size and superior mechanical longevity [11]. The direction in which the blood enters and leaves the impeller of the Heartmate II is termed axial flow. Axial flow rotary blood pumps are small and contain an impeller that can spin at speeds between 6000 and 15,000 rotations per minute to deliver blood flow to the circulatory system [11]. Suitable hemodynamic pressures and flows are typically produced at about 9000 rotations per minute [11]. The pump lies parallel to the diaphragm and may be placed within the muscles of the abdominal wall. The only part that moves on the pump is the rotor and it spins on inlet and outlet ball-and-cup bearings. The motor of the device creates a spinning magnetic field that spins the rotor and imports torque to its internal cylindrical magnet. Blood flows from the inlet tube into the pump, past three airfoilshaped guide vanes that straighten the flow of blood as it is picked up by the rotor. The rotor consists of three curved blades. As the rotor spins, kinetic energy is supplied to the blood flow as it moves toward the outlet vanes and into the aorta where it embarks on its journey through the circulatory system [11]. Since the approval for bridge to transplant in 2008, and destination therapy in 2010, more than 5000 patients worldwide have been implanted with the Heartmate II [12]. After the initial surgery to implant the device, 86% of the participants with the Heartmate II and 76% of people with the Heartmate XVE were released from the hospital after about twenty-seven days. Throughout the trial, patients with the Heartmate II spent 88% of the time outside the hospital as opposed to 74% of the Heartmate XVE. All the patients were tested at regular intervals to judge their health and quality of life. A six-minute walk test was done at one month, three months, and then every six months after until the end of the trial to study the patient’s energy and strength [12]. After two years, eighty percent of the patients who survived with the Heartmate II had only stage one or stage two NYHA symptoms and could double their six-minute walk distance of two years prior. Questionnaires were distributed to all of the surviving patients to describe their quality of life with the devices. Results of the questionnaires showed a much higher improvement of quality of life with the Heartmate II rather than the Heartmate XVE [12]. After the entire trial was over, results of the Heartmate II continuous flow left ventricular assist device were compared the results of the pulsatile flow Heartmate XVE. The Heartmate II proved to be superior to the Heartmate XVE. The Heartmate II achieved higher percentages of primary end points and actual survival rates, better quality of life assessments, and lower cases of adverse events like stroke, right heart failure, respiratory failure, and renal failure. The durability of the Heartmate II was exceedingly better than the Heartmate XVE. Only six out of every 100 Heartmate II’s needed replacement compared to forty-eight out of a 100 for the Heartmate XVE [12]. This trial’s results thoroughly detailed the preeminence of the continuous flow Heartmate II. Clinical Results The continuous flow Heartmate II LVAD was placed in a trial with the pulsatile flow Heartmate XVE LVAD to compare the Heartmate II’s clinical results with a device that has been previously tested. The Heartmate XVE has already proven that it increases the quality of life and survival rates compared to medical therapy alone. Throughout the trial, the Heartmate II surpassed the Heartmate XVE in almost every way [12]. The trial consisted of two hundred patients with advanced heart failure randomly being assigned to two groups. The first group contained one hundred and thirty-four patients receiving the Heartmate II, and the second group had sixtysix patients being implanted with the pulsatile flow Heartmate XVE. Due to its smaller size, more women were placed into the Heartmate II group. The median age of the patients was 64 years old, with ages ranging from twenty-six to eighty-one years of age. Every trial has primary and secondary endpoints that they use to determine the effectiveness of the device that is being studied. In this trial, the primary endpoint was two years of use of the device without any incapacitating stroke, operation to fix or replace the device. Secondary endpoints included quality of life, survival, and functional capacity. By the end of the trial, 46% of the patients on the Heartmate II achieved the primary endpoint as opposed to only 11% of the patients on the Heartmate XVE. The Heartmate II had over double the actual survival rate of the Heartmate XVE, at 58% [12]. Comparability What separates the Heartmate II LVAD from LVADs of the past is the continuous flow system that is utilized. Previous devices contained a pulsatile flow. The pulsatile output closely simulates the ventricular action of the heart. It provides physiological advantages in blood flow for perfusion in cardiovascular and hemodynamic studies [10]. The pulsatile flow was favored in the medical community for years, and when the smaller-sized continuous flow devices surfaced, there was initial skepticism [13]. The skepticism concerned the long-term physiological effects of a ‘non-pulsatile’ pump, such as the formation of a blood clot [10]. A study was conducted comparing the results of pulsatile flow rotary pumps versus continuous flow rotary pumps. The primary outcome showed that the survival free from stroke or reoperation to repair or replace the device was 46% for the continuous flow group and 11% for the pulsatile flow group [12]. Both types of devices improve the quality of life and functional capacity, but the continuous flow LVAD improved the probability of survival free from stroke and 3 Patrick Devlin Patrick Bianconi device failure at two years compared with a pulsatile device [11]. Another advantage of the continuous flow pump is that it is smaller and thus requires less surgical dissection for implantation [11]. The continuous flow group in the study had about half the amount of device-related infections in patients than in the pulsatile flow group [11]. Continuous flow rotary pumps can be further specified into axial flow or radial flow. As stated before, this is the method in which the blood enters and leaves the impeller of the device [10]. In axial flow pumps, the fluid flows spirally along the axis and is nicknamed the “propeller pump”. In radial flow pumps, the fluid flows outward at a right angle to its axis. The Heartmate II utilizes the axial flow pump, and as stated before this axial pump is very small in size, can be implanted in a wide range of patients, and can spin at speeds between 6000 and 15,000 rotations per minute [10]. The radial, or centrifugal, flow pump is slightly larger in diameter and spins at slower speeds than the axial pump. These radial pumps are suitable for long-term cardiac assistance due to the lower rotational speed and higher hydraulic efficiency [11]. Both types of pumps provide full circulatory support and can be implanted in such a way that will aide the heart as a bridge to transplant or even as destination therapy. Devices that use the radial flow pump are currently undergoing clinical trials for FDA approval in the United States. The future looks bright for radial flow in devices, but approval by the FDA must happen first, and when/if it does, the Heartmate II could possibly utilize this method of flow [11]. Until the approval of radial flow, axial flow remains to be the most reliable and efficient way to direct the flow of blood in LVADs. This allows for the assertation to be made that the Heartmate II is currently the best LVAD available on the market. across the room. Patients with the Heartmate II have reported significantly more energy even after only a few months of receiving the machine. After recovering from surgery, the patient is able to go about their day almost as if they did not have heart problems. People have been reported to participate in snowboarding and other sports with the Heartmate II, proving that the machine can provide a level of energy similar to that of a healthy heart. The quality of life for a patient, without the Heartmate II, who barely has the energy to walk is far inferior compared to a patient with the Heartmate II who can play sports [14]. In the NPSE code of ethics for engineers, one of the canons states that engineers must work for the safety, health, and welfare of the public [15]. The Heartmate II gives patients an opportunity to not only leave the hospital, but also enough energy to go about the day as though they have a normal, healthy heart. An obvious positive aspect to the patient’s health is that the patient’s heart is working at full capacity. A not so obvious positive aspect that also comes with the Heartmate II is the psychological health benefits that come hand in hand with living a normal healthy life outside of the hospital. Patients can experience a typical day with their friends and family that they would never have the opportunity to experience if it wasn’t for the Heartmate II. The importance of psychological health should never be underestimated. Some may argue that it is as important as physical health. Artificial organs are a controversial topic. Some people do not like the idea of people living with artificial organs that are basically machines. Many cases from the past have involved intense debates regarding the ethical nature of a person being kept alive by a machine. For instance when a patient who has suffered brain damage is put into a permanent vegetable like state, that person requires machines to feed, control their breathing, and other functions that they would not be able to sustain life without. In those cases, the patients themselves are in a state which they cannot make the decision for themselves. This raises more ethical issues as to who has the power to decide what is best for them. In the cases involving the Heartmate II however, the patient has the ability to decide if the machine is right for them. Also, left ventricular assist devices are only assisting the patient’s existing, normal heart. This is very similar to the way that an inhaler assists the lungs of a person with asthma. The Heartmate II has also been known to be used as a method of myocardial recovery. Myocardial recovery means the machine slowly helps the natural organ begin to function on its own again. In some cases , the Heartmate II has strengthened the ventricles of the heart enough that they had begun to work properly on their own without the aid of the LVAD. Ideally, this would be the best possible outcome, but sadly, it does not happen nearly enough. PROBLEMS OF THE REALITY IN RETROSPECT Heart disease is currently plaguing the lives of millions of people and hundreds of thousands more each year. Clearly, new technologies and advancements must be made to fight this horrible disease. Biomedical engineers have been working to find solutions to save millions from heart related problems. The first goal for engineers is to design a device that will keep the patient with heart failure alive. This used to require constant hospitalization with no hope of leaving the hospital and experiencing a normal day. Engineers began to develop artificial hearts and ventricular assist devices in hopes of giving patients a chance at a normal life again. Total artificial hearts are slowly becoming capable of discharging patients from the hospital, but not nearly at the capacity of LVADs. The Heartmate II is the best left ventricular assist device on the market to give a patient the opportunity for a normal life. Heart Disease has been known to drastically reduce the energy of sufferers. In most cases, patients barely have the energy to get out of bed and walk Common Issues 4 Patrick Devlin Patrick Bianconi The Heartmate II is an incredible device that has the potential to save millions of lives, but like almost every machine that has been designed, it has flaws. Users of the Heartmate II, and all continuous-flow VADs for that matter, will appear to not have a pulse. The user must carry around documentation indicating that the lack of a pulse does not mean the person is dead. Other than being rather eerie, this problem does not affect the well-being of the patient. In a normal heart, there is a balance between the amount of blood the right ventricle supplies to the left ventricle in order to be distributed throughout the body. With an LVAD in place, Right Ventricular Failure has the potential to occur. This happens because the right ventricle stops working properly and does not provide an adequate reservoir of blood to the left side, so the LVAD does not have a sufficient amount of blood to pump. Another possible issue with LVADs occurs when the device is pumping blood at too fast of a pace and the device becomes displaced thus preventing proper functioning. This is called Cannula Obstruction. As a possible result of Cannula Obstruction and as a pre-cursor to Right Ventricular Failure, Arrhythmias involves unnatural rhythmic blood flow. Normally the left ventricle can compensate for the unbalance, but the right ventricle can’t handle it in comparison with the LVAD in place. This then leads to Right Ventricular Failure. One factor that can negatively contribute to the success of the device is the clotting of blood in the bearing of the device that leads into the left ventricle. These clots can build up in the bearing and break off and flow into the aorta, which in turn can lead to a stroke by cutting off proper blood supply. Similar to this is aortic stenosis, where calcium deposits build up in the aorta thus increasing the pressure in the cardiovascular system which can also lead to a stroke and even gastrointestinal bleeding [13]. Possibly the largest problem with the Heartmate II and many of the other LVADs on the market is the percutaneous drive line that supplies power to the device. This constant exposure to the wound can lead to a wide range of serious infections that can in turn plague the user of the device. It is also of utmost importance that special care and attention is paid to the open wound, because if it is bumped, the power supply can potentially get cut off and the open wound can be harmed, leading to infection. One of the more limiting factors of the Heartmate II, is the amount of strenuous activity the user’s heart and LVAD can handle. A manual control system is in place to allow the user to operate the speed at which the impeller of the device rotates. This seemingly helpful function can lead to serious consequences if not properly operated by the user [14]. The Heartmate II is not a cheap machine when used as a bridge to transplantation. The LVAD costs around $150,000 plus more bills for hospitalization and drugs. After receiving the Heartmate II, patients are discharged from the hospital after about one month and are capable of staying out of the hospital for long amounts of time if no adverse events occur. Hypothetical, with its ability to keep the patient out of the hospital for long periods of time, the Heartmate II could save money if used as destination therapy. The device is not used long enough as a bridge to transplantation to save money on hospital bills that would accumulate if the patient stayed in the hospital as opposed to receiving the device. Although cost may be an issue when deciding to use the Heartmate II, the survival rates and improvement of quality of life that come with the machine outweigh the monetary cost of the device [16]. A price tag cannot be put on the positive psychological effects of living a normal life outside of the hospital. THE FUTURE OF ARTIFICIAL HEART TECHNOLOGIES Biomedical engineers are constantly designing new products that are far superior to their predecessors. Creating new, more effective artificial organs is a process much like designing a building or even writing a paper. Each time a device is created, it is the best version of the idea, but newer, more efficient models will eventually be designed. Devices relating to failing hearts are no exception. As stated before, the current top model of left ventricular assist devices is Thoratec’s Heartmate II. This is the only device that is FDA approved for both bridge to transplant and destination therapy. The approval backs the reliability of the device, and clinical results have agreed as well. The success of Thoratec’s device should inspire the company to continue making improvements and come out with newer, more efficient models that will greater improve the quality of life of a heart failure patient. Currently undergoing design is the Heartmate III and the Heartmate X. These devices hold promising outcomes in the future of artificial heart technologies. The devices aim to make such improvements as the reduction of clotting, a smaller pump, and a less of a requirement of energy, enabling the battery life to last longer. The newer devices will hopefully contain a transcutaneous power source, rather than having a driveline connect to the external power source through an open wound in the side of the patient. This will eliminate the chance of infections entering the body through that wound [8]. As time continues to change, and new advancements are made in the medical and technological fields, further improvements can be made to any future artificial heart device. Currently, the Heartmate II LVAD reigns at the top of the ventricular assist device totem pole, supplying the weakened heart with the appropriate aide to pump blood throughout the body. With such a complex device dealing with such a complex problem, the Heartmate II may have its flaws. The improvement of the quality of life, compared to life without the device, however, fully compensates for these flaws. All in all the Heartmate II is affecting the lives of 5 Patrick Devlin Patrick Bianconi Device.” The New England Journal of Medicine. [Online]. Available: http://www.nejm.org/doi/full/10.1056/NEJMoa0909938 [13] K. Shah, D. Tang, and Richard Cooke. (2010, March 23). “Implantable Mechanical Circulatory Support: Demystifying Patients With Ventricular Assist Devices and Artificial Hearts.” Wiley Online Library. [Online]. Available: http://onlinelibrary.wiley.com/doi/10.1002/clc.20825/full; [14]Weiss, W. (2012). Personal Interview. [15] (2007, July) “NSPE Code of Ethics for Engineers.” NSPE.org [Online: Article]. Available: http://www.nspe.org/Ethics/CodeofEthics/index.html [16] (2011) Moreno. “Cost-effectiveness of the implantable Heartmate II left ventricular assist device for patients awaiting heart transplantation.” Pubmed. [Online]. Available: http://www.ncbi.nlm.nih.gov/pubmed/22115674; thousands and will continue to advance as a leading device in fighting heart failure, thus improving the lives of millions. REFERENCES [1] Cheesman, Kim, Ortiz, Miller (2010) “Heartmate II” Heart Failure Online [Online]. Available: http://www.heartfailure.org/eng_site/whats_new_heartmate_ ii.asp [2] (2008, May 2) “FDA Approves Heartmate II Mechanical Heart Pump for Heart-failure Patients” Biology News Net [Online]. Available: http://www.biologynews.net/archives/2008/05/02/fda_appro ves_heartmate_ii_mechanical_heart_pump_for_heartfailure_ patients.html [3] Adrian F. Hernandez, G. Michael Felker. (2011, September-October). “Advanced Heart Failure.” Duke Clinical Research Institute. [Online]. Available: http://www.sciencedirect.com/science/article/pii/S00330620 11001344 [4] Rohinton J. Morris, MD. (2008, November 28). “Total Artificial Heart—Concepts and Clinical Use.” Seminars in Thoracic and Cardiovascular Surgery. [Online]. Available: http://www.sciencedirect.com/science/article/pii/S10430679 08001032 [5] (2011, January 1). “Ventricular Assist Devices and Total Artificial Hearts.” Regence. [Online]. Available: http://blue.regence.com/trgmedpol/surgery/sur52.html; [6] S. Willets (2008, Dec 15) “History of Ventricular Assist Devices” The Thoratec Heartmate II Advisor [Online] Available: http://www.heartmateadvisor.com/history [7] Reed Miller. (2010, January 20) “FDA approves Thoratec’s Heartmate II LVAD for destination therapy” Heartwire [Online] Available: http://www.theheart.org/article/1041407.d [8] (2011, January 11). “A New Era in the Treatment of Advanced Heart Failure” Thoratec Corporation. Pgs. 5-20 [9] (2011) “Heartmate II” MUSC Health Heart and Vascular Center [Online]. Available: http://www.muschealth.com/heartmate [10] R. Starling, Y. Naka, A. Boyle, G. Gonzalez-Stawinski. (2011). “Results of the Post-U.S. Food and Drug Administration-Approval Study With a Continuous Flow Left Ventricular Assist Device as a Bridge to Heart Transplantation.” Journal of the American College of Cardiology. [Online]. Available: http://www.sciencedirect.com/science/article/pii/S07351097 11007352 [11] Daniel Timms. (2011, November). “A review of clinical ventricular assist devices.” Medical Engineering & Physics. [Online]. Available: http://www.sciencedirect.com/science/article/pii/S13504533 11000919 [12] Mark S. Slaughter, Joseph G. Rogers, Carmelo A. Milano. (2009, December 3). “Advanced Heart Failure Treated with Continuous-Flow Left Ventricular Assist ADDITIONAL RESOURCES (2012, January 27) “America’s Heart Disease Burden” National Center for Chronic Disease Prevention and Health Promotion. [Online] Available: http://www.cdc.gov/heartdisease/facts.htm (2012) “Heartmate II Left Ventricular Assist System” Thoratec Corporation [Online]. Available: http://www.thoratec.com/medical-professionals/vadproduct-information/heartmate-ll-lvad.aspx (2011) “Questions about HF.” Heart Failure Society of America. [Online]. Available: http://www.abouthf.org/questions_stages.htm; (2010) “The SynCardia Total Artificial Heart: What to Expect” SynCardia Systems Inc. [Online]. Available: http://www.syncardia.com/Patients-and-Caregivers/thesyncardia-total-artificial-heart-what-to-expect.html ACKNOWLEDGMENTS We would like to thank Dr. William Weiss, the Engineering 0012 professors, the writing instructors, the reader who is reading this, and most important of all, the suffers of heart failure for fighting through their tough times. 6