JMJ Chemical Reactions Theme: Changes and Reactions Physical
advertisement

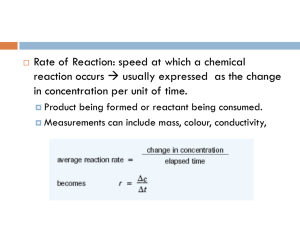
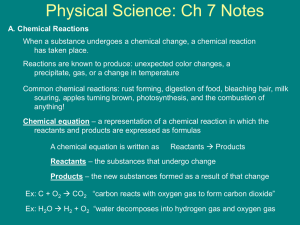
JMJ Chemical Reactions Theme: Changes and Reactions Physical Properties (Review) Color, melting point, boiling point, electrical conductivity, specific heat, density, state (solid, liquid, or gas) Physical Change (Review) Changes in physical properties - melting, boiling, condensation, freezing No change occurs in the identity of the substance Chemical Change Atoms in the reactants are rearranged to form one or more different substances Old bonds are broken; new bonds form Main Ideas Chemical Reactions are represented by Chemical Equations. Chemical Equations are balanced to show the same number of atoms of each element on each side. The Law of Conservation of Matter (Mass) says that atoms won’t be created or destroyed in a chemical reaction. That is why chemical equations must be balanced! Vocabulary Reactants Products Chemical Formula Chemical Equation Coefficients Chemical Reaction A process in which at least one new substance is produced as a result of chemical change. How do you know when a chemical reaction takes place? Color Change, Precipitate Formation, Gas Formation, Odor, Temperature Change, Change in Acidity Representing Chemical Reactions – Chemical Equations Chemical Equations Reactants produce Products Reactants Products Solid Sodium combines with Chlorine gas to make solid Sodium Chloride: 2Na (s) + Cl2 (g) 2NaCl Reactant A + Reactant B Product The reactants are used up in forming the product The arrow shows the direction of the reaction Writing a Chemical Equation Chemical symbols give a “before-and-after” picture of a chemical reaction Law of Conservation of Matter (Mass) Matter cannot be created or destroyed. However, it can change form. The mass of the reactants must equal the mass of the products in a chemical reaction. Balancing Chemical Equations The total number of each type of atom must be the same on both sides of the equation. Understanding Chemical Formulas Subscripts BaF2 the 2 in this formula is called the subscript. It refers only to the element preceding it. In this case the F (fluorine). Parentheses Al(NO3)3 in some chemical formulas it is necessary to use parentheses. The subscript outside the parentheses refers to all the elements inside the parentheses. In this example there are: one Al (aluminum), three N (nitrogen), and nine O (oxygen). Coefficients 3 BaF2 the 3 in this formula is called the coefficient. It refers to each element that follows. In this case there would be 3 Ba (barium) and 3 F2 (a total of 6 fluorine). Reaction Rates Increasing Temperature increases reaction rate Surface Area – increasing the surface area increases the rate of reaction. Concentration – amount of reactants in a given volume. Stirring increases reaction rate Catalyst – speeds up a reaction but does not change during the reaction Catalysts do not get used up in a reaction HCl CH3COCH3 + I2 CH3COCH2I + HI HCl is a catalyst, necessary for the reaction but not used up in the reaction, that is why it is written above the reaction arrow. Increasing reactants increases the rate of reaction and increases the products made Reactions will occur until at least one of the reactants is all used up Mass of all the reactants is equal to the mass of all the products Spectrophotometer measures absorbance or transmission of light Can be used to measure the disappearance or appearance of products or reactants in a chemical reaction Summary As the amounts of the reactants increase, the amounts of the products produced also increase. In Investigation 1, as the amounts of the NH4OH and the HCl increased, the amount of heat produced increased. Reactants must be present in the reaction in equivalent amounts to produce the maximum amount of all products. In Investigation 2, the HCl was present in a greater amount than the magnesium since all the magnesium was consumed by the reaction to produce H2 gas. When the amount of magnesium was increased from 0.5 to 1 gram, the excess HCl was able to react and produce more H2. No matter is lost from a chemical reaction even if the reactants are not present in equivalent amounts. Every atom of every reactant can be found in either the products or in 2 reactants that are not consumed. In Investigation 2, the atoms of the HCl were found either in the unreacted HCl or in the H2 produced. In every reaction of the Investigation, all of the magnesium atoms were converted to the product, MgCl2. As the amount of a reactant increases, the rate of the reaction increases. The rate can be measured either as an increase in the rate of disappearance of a reactant or an increase in the rate of production of a product. Therefore, more reactant added to a reaction, results in more products produced at a faster rate. Investigation 3, as the concentration of the reactant acetone increased, the reaction rate increased as evidenced by the more rapid disappearance of the colored reactant, I2. 3