jane12306-sup-0001
advertisement

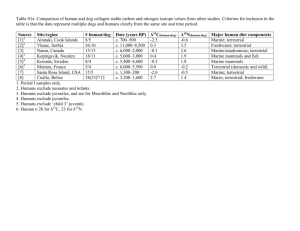
1 Supp. 1: 2 Stomach content analysis 3 Number of individual prey items (i.e., numerical counts) was assigned based on the 4 presence of species-specific body parts known to represent one individual for a particular taxon. 5 All prey taxa were first identified to the lowest taxonomic subdivision possible, in most cases 6 genus or species, and then categorized into resource groups according to the prey species’ known 7 habitat use patterns and presence in specific habitats during stable isotope sampling. Prey were 8 classified into either marine/estuarine (species which inhabit marine/estuarine habitats as well as 9 those that are known to consume resources from both marine/estuarine and freshwater/upland 10 habitats) or freshwater/upland (species which inhabit freshwater wetlands or terrestrial upland 11 habitats) resource categories. Cross-ecosystem foragers, Procyon lotor Linnaeus 1758 12 (Procyonidae, raccoon) for example, were classified as marine/estuarine since these species often 13 obtain a large portion of their dietary resources from marine/estuarine ecosystems (Fleming, 14 Palisano & Joanen 1969). 15 16 Stable isotope sample collection and analysis Crocodilian scutes (superficial scale-like structures of the skin) are chiefly composed of 17 two biological materials, keratinous epidermal layer and cartilaginous dermal layer comprised of 18 dense collagen fibers (Alibardi & Thompson 2000; Alibardi & Toni 2007). Frozen whole caudal 19 scute samples were thawed, cleaned with deionized water and gently hand washed to remove 20 foreign debris (i.e., epiphytic algae and detritus). After cleaning, the keratinous epidermal layer 21 of the scutes was separated from dermal collagen layer using NaOH digestion (Radloff, Hobson 22 & Leslie 2012) or mechanically using a sterile scalpel. Isolated keratin samples were dried for 23 48 hours at 60 °C and ground. Alligator mississippiensis scute tissue has a slow rate of turnover 24 (590 days for δ13C and 414 days for δ15N, Rosenblatt and Heithaus 2013), thus stable isotope 25 signatures of scute keratin represent incorporation of long-term dietary patterns (> 1 year). 26 Representative primary producer and potential prey species were sampled 27 opportunistically from fall 2007 to summer 2009 at five sites within upland freshwater habitats 28 and five sites within marine/estuarine habitats (Fig. 1). If samples could not be collected, values 29 were obtained from literature sources. For primary producers, aggregate samples consisted of 30 live green leaves collected from 10 to 15 individual plants or live clumps in the case of 31 macroalgae species. All samples were placed on ice at the time of collection and frozen at -10 32 °C until further processing. Samples were washed with deionized water, gently rubbed free of 33 debris, dried at 60 °C for 48 hours, and ground. 34 Representative prey items and food web constituents were sampled using dip-nets, 35 minnow traps, seine nets, single-line sampling, collected from roadkill, and harvested by local 36 recreational hunters and fishermen. Smaller organisms (< 1cm) were aggregate samples 37 consisting of 10-15 whole individuals. For larger animals, 1 to 3 g of bulk muscle tissue was 38 taken from a single specimen. Animal samples were cleaned with deionized water, indigestible 39 body portions removed (i.e., carbonate shell, scales, hair), dried at 60 °C for 48 hours or until 40 completely dehydrated and ground. 41 Approximately 500 to 800 µg of homogenized animal tissues or 1 to 3 mg of plant tissues 42 was weighed and loaded into 9 × 5 mm tin capsules for stable isotope analysis at the University 43 of Florida Geology Stable Isotope Laboratory, Gainesville, Florida. Analyses were performed 44 using one of two systems: either a Finnigan DeltaPlus XL isotope mass spectrometer with 45 ConFlo III interface linked to a Costech ECS 4010 Elemental Combustion System (elemental 46 analyzer) or Finnigan-MAT 252 isotope ratio mass spectrometer coupled with a ConFlo II 47 interface linked to a Carlo Erba NA 1500 CNS Elemental Analyzer. Stable isotope values are 48 expressed in standard per mil notation δX (‰): δX (‰) = [Rsample⁄Rstandard -1 ]×1000, where X is 49 the element of interest and R is the ratio of heavy to light isotopes (13C/12C or 15N/14N) of the 50 sample and standard (Vienna Pee Dee Belemnite used for δ13C and Atmospheric Nitrogen-AIR 51 for δ15N). Machine accuracy was measured and corrected for during each sample run, using four 52 measures of in-lab standard USGS-40 (l-glutamic acid, δ13C = -26.39 and δ15N = −4.52) with 53 analytical machine error for USGS-40 was 0.12‰ ± 0.04 for δ15N and 0.1‰ ± 0.05 δ13C (mean ± 54 SD). 55 References 56 57 58 Alibardi, L. & Thompson, M.B. (2000) Scale morphogenesis and ultrastructure of dermis during embryonic development in the alligator (Alligator mississippiensis, Crocodilia, Reptilia). Acta Zoologica, 81, 325–338. 59 60 61 Alibardi, L. & Toni, M. (2007) Characterization of keratins and associated proteins involved in the corneification of crocodilian epidermis. Tissue and Cell, 39, 311–323. 62 63 64 Fleming, D.M., Palisano, A.W. & Joanen, T. (1969) Food Habits of Coastal Marsh Raccoons with Observations of Alligator Nest Predation. Wildlife Session pp. 348–357. U.S. Fish and Wildlife Service. 65 66 67 Radloff, F.G.T., Hobson, K.A. & Leslie, A.J. (2012) Characterising ontogenetic niche shifts in Nile crocodile using stable isotope (δ13C, δ15N) analyses of scute keratin. Isotopes in Environmental and Health Studies, 48, 439–56. 68