Alcohols, Ethers, Thiols Worksheet
advertisement
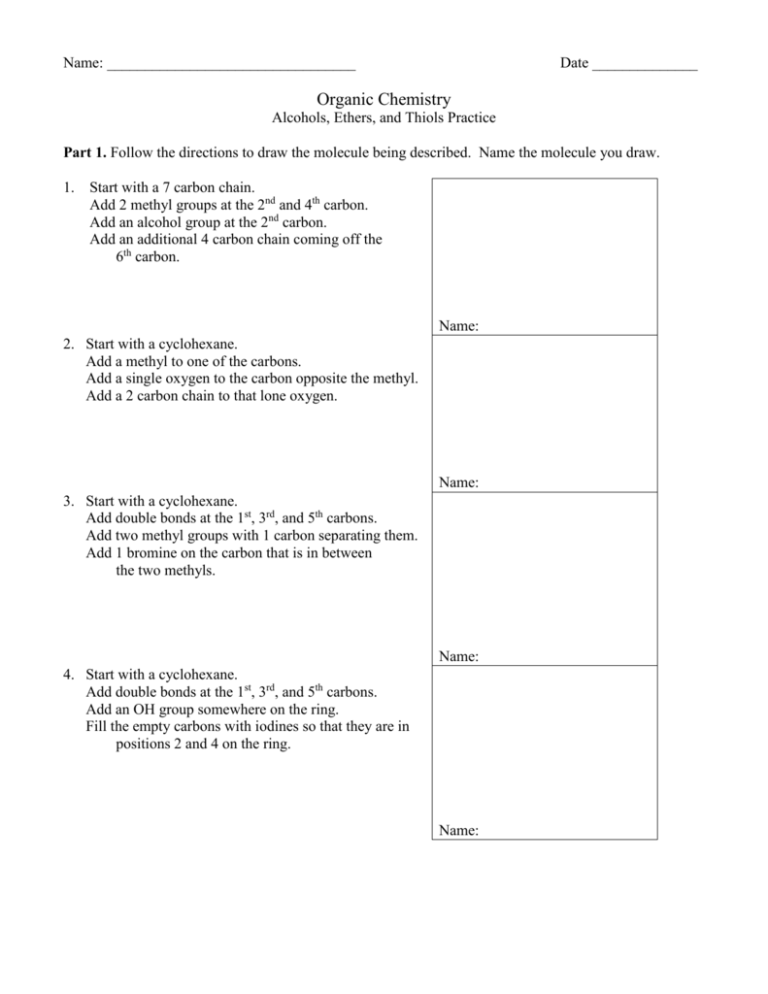
Name: _________________________________ Date ______________ Organic Chemistry Alcohols, Ethers, and Thiols Practice Part 1. Follow the directions to draw the molecule being described. Name the molecule you draw. 1. Start with a 7 carbon chain. Add 2 methyl groups at the 2nd and 4th carbon. Add an alcohol group at the 2nd carbon. Add an additional 4 carbon chain coming off the 6th carbon. Name: 2. Start with a cyclohexane. Add a methyl to one of the carbons. Add a single oxygen to the carbon opposite the methyl. Add a 2 carbon chain to that lone oxygen. Name: 3. Start with a cyclohexane. Add double bonds at the 1st, 3rd, and 5th carbons. Add two methyl groups with 1 carbon separating them. Add 1 bromine on the carbon that is in between the two methyls. Name: 4. Start with a cyclohexane. Add double bonds at the 1st, 3rd, and 5th carbons. Add an OH group somewhere on the ring. Fill the empty carbons with iodines so that they are in positions 2 and 4 on the ring. Name: Part 2: All of the following alcohol molecules have the same basic structure. The only thing that changes each time is the placement of the –OH group. Name each molecule. HO HO Name: ___________________________ Name: _______________________________ OH Name: ____________________________ OH Name: _______________________________ Part 3: Name the following ethers. Use –oxy if groups on either side of –O—are too complicated. O O Name: ____________________________ Name: _______________________________ O O Name: ____________________________ Name: _______________________________ O Name: ____________________________ O Name: _______________________________ Part 4: Draw the following molecules. MOST are thiols and phenols. Draw: 3-octanethiol Draw: 2,3-diiodophenol Draw: 1-ethoxy-2-butanethiol Draw: 2-methylbenzenethiol Draw: 1,2-dimethyl 3-chloro-5-propylphenol Draw: 3-cyclobutylphenol Part 5: Predict the products of the dehydration reaction below AND name them. H H OH H H H C C C C C H H H CH3 H H + Part 6: For each name listed below, there is a better and more correct name. Write the “more correct” name on the indicated line. Example: 5-methyl phenol Should be: 1-methyl phenol 2,4,4-trimethyl-5-pentanol Should be: ____________________ 1,3,4-cyclohexatriol Should be: ____________________ 5-octanol Should be: ____________________ methyl ethyl ether Should be: ____________________ ethoxymethane Should be: ____________________ 3,5-dithioloctane Should be: ____________________ 2,5,6-trimethylphenol Should be: ____________________ Part 7: Hydrogen bonding. Draw diagrams below illustrating how hydrogen bonding occurs between two water molecules, two ethanol molecules and two methoxy methane molecules. One of the three diagrams will not be able to show H-bonds. Label that diagram with “No H-bonds Possible” H-bonding in water H-bonding in ethanol H-bonding in methoxy methane Part 8: Name the following organic molecules. They may be alkanes, alkenes, alkynes, alcohols, ethers, thiols or phenols. Name: ____________________ Name: ___________________ Name: __________________ Name: ____________________ Name: ___________________ Name: ___________________ Name: ____________________ Name: ____________________ Name: ___________________ Name: ____________________ Name: _____________________ Name: ___________________ Part 9: Draw the following. Phenoxybenzene 2-cyclopropyl-3-heptanol cyclopentoxyoctane