Lesson 1 Lecture Notes
advertisement

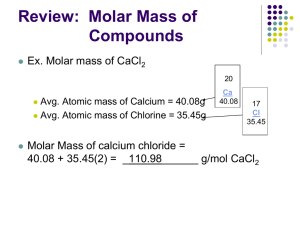
Avogadro’s Hypothesis and the Mole Avogadro’s Hypothesis Recall from Unit 3 that John Dalton theorized how atoms combine to form molecules. He assumed that hydrogen was the lightest element and assigned it an arbitrary mass of 1 and so if oxygen weighed 16 times as much as hydrogen, it would have a relative mass of 16. There are two reasons why Dalton’s method were faulty. First, we now know that atoms of the same element can have different masses. Second, he assumed that every compound contained only 1 of each atom so different ratios were not accounted for. At around the same time, French chemist Joseph Gay-Lussac experimented with pairs of gases at the same temperature and pressure and found precise ratios of reactants based on volume. E.g. 1 L of hydrogen gas reacts with 1 L of chlorine gas to make 2 L HCl (g) 1 L of nitrogen gas reacts with 3 L hydrogen gas to make 4 L of NH3 (g) Italian chemist Amadeo Avogadro proposed the following explanation for Gay-Lussac’s data. Avogadro’s Hypothesis: equal volumes of different gases at the same temperature and pressure contain the same number of particles. This means that 1 L of hydrogen gas contains the same number of atoms as 1 L of chlorine gas and that 1 molecule of hydrogen gas reacts with 1 molecule of chlorine gas. Avogadro was able to connect the volume, mass and the number of particles of chemicals. The Mole A mole is the number of carbon atoms in exactly 12 g of 12C which is experimentally measured to be 6.022 x 1023 called Avogadro’s Number or Avogadro’s Constant. It is also the number of particles (atoms or molecules) in one mole of any element or compound There are no units for Avogadro’s Number because it is simply a number just like a dozen is 12 and a pair is 2. We can write a conversion factor for mole just like we did for dozen and pair: 12__ __2__ 1 dozen 1 pair 6.022 x 1023 1 mole However, unlike the first two, Avogadro’s Number is a measured number not an exact number so its significant figures must be accounted for in calculations. Practice: How many molecules are there in 0.125 mol of molecules? How many moles of H2O are there in 54.478 x 1023 molecules? Molar Mass The molar mass is the mass of one mole of (6.022 x 1023) particles. The molar mass of an element is the same as the atomic mass shown on the periodic table, expressed in grams. To find the molar mass (mass per mole) of a monatomic element, simply look at the periodic table. For the molar mass of a compound, we would need to add the molar mass of every atom in the compound together. Example: Find the molar mass of a molecule of H2O. H2O has 2 hydrogen atoms with a molar mass of 1.0079 g each and an oxygen atom with a molar mass of 15.9994 g Molar mass of H2O = 2(1.0079 g) + 1(15.9994 g) = 18.0152 g/mol Practice: Find the molar masses of C12H22O11 and (NH4)2SO4 Once we find the molar mass of an element or compound, we can use it as a conversion factor for converting between moles and mass. Practice: How many moles of H2O are there in 60.00 g? How many grams in 2.25 mol? Converting Between Particles, Moles and Mass We have just learned how to convert between particles and moles using Avogadro’s Number as well as converting between moles and mass using molar mass. Now, we can connect the two together and convert between particles and mass by using both conversion factors. Practice: How many grams of Ca(OH)2 are there in 13.495 x 1023 molecules? How many molecules of nitrogen gas are there in 30.491 grams? Mole Y Diagram Fill in the conversion factors needed to “mole-tiply” and convert between mole, mass, volume and particles.