Chap. 10 Mole Conversions
advertisement
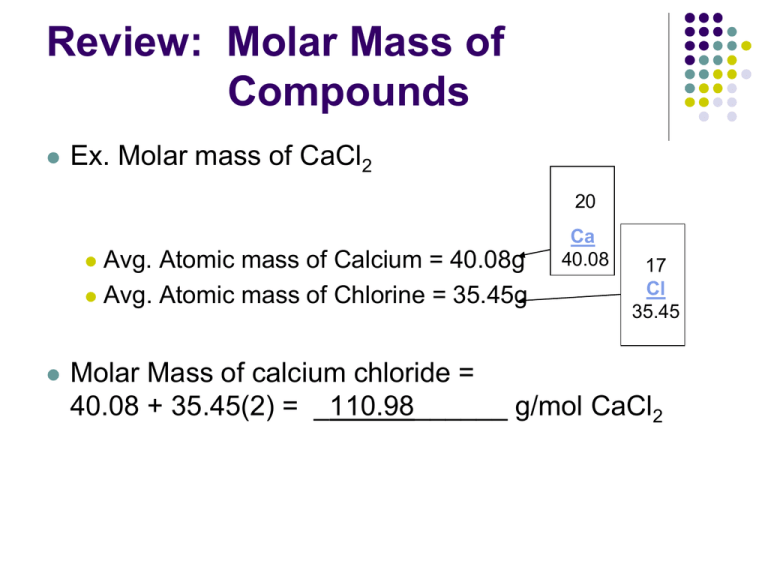
Review: Molar Mass of Compounds Ex. Molar mass of CaCl2 20 Avg. Atomic mass of Calcium = 40.08g Avg. Atomic mass of Chlorine = 35.45g Ca 40.08 17 Cl 35.45 Molar Mass of calcium chloride = 40.08 + 35.45(2) = _110.98______ g/mol CaCl2 Practice Calculate the Molar Mass of calcium phosphate Formula = Ca3(PO4)2 Masses elements: Ca = 40.08g/mole P = 30.97 g/mole O = 16.00 g/mole Molar Mass = (40.08 g/mole)(3) + (30.97 g/mole)(2) + (16 g/mole)(8) = 310.18 g/mole Mole Conversions Learning Target: Understand how molar mass relates to the number of moles and particles of a substance. Particles (atoms, Formula units, molecules) Divide by 6.02 X 1023 Multiply by 6.02 X 1023 Moles Multiply by molar mass from periodic table Divide by molar mass from periodic table Mass (grams) 3.5 dozen roses = ?? Roses 2.5 mol roses = ?? Roses (use Avogadro's #) Problem Type #1 – Moles to Mass Find the mass of 0.760 mol of magnesium bromide.. Find the molar mass of the compound: Make the conversion: Problem Type # 2– Mass to Moles A bottle of copper (II) nitrate contains 110.6 g of compound. How many moles of copper (II) nitrate are in the bottle? Problem Type #3 – Moles to Particles Determine the number of atoms that are in 0.78 mol of mercury. Problem Type #4 – Particles to Moles How many moles of zinc sulfate contain 5.40 x 1024 formula units? Multi-step Mole Conversions 2.5 g Roses = ?? atoms of roses Atomic mass of 1 Rose is 3.0 g/mol Multi-step Practice How many molecules are in 7.4 g of potassium phosphate? Multi-step Practice How many grams of sodium bicarbonate are in 1.8 x 1023 formula units? Molar Volume Definition: The volume of one mole of an ideal gas at standard conditions (STP) equal to 22.4 L. STP = standard temperature and pressure which is 0ºC and 1 atmospheric pressure (760 mmHg) 1 mole gas at STP = 22.4 L Particles (atoms, Formula units, molecules) Divide by 6.02 X 1023 Multiply by 6.02 X 1023 Moles Multiply by 22.4 L Divide by 22.4 L Volume (L) Practice A container with a volume of 563L contains how many moles of air at STP? How many molecules of air? Practice A chemical reaction produced 0.78 mol of H2 gas. What volume will the gas occupy at STP? How many molecules of H2? How many atoms of hydrogen?