Quantum Number and Electronic Configuration Notes
advertisement

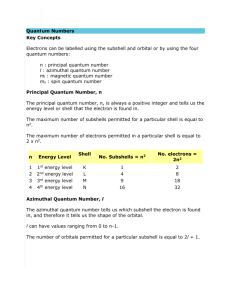
Quantum Mechanical Theory - Allows us to predict the probability of finding an electron at various points in space Allows us to explain both the atomic spectra of elements better, the arrangement of elements on the periodic table of elements, and understand chemical bonding Bohr’s theory Term used: orbits 2-D path Fixed distance from the nucleus Quantum Mechanical theory Term used: orbital 3-D region in space Variable distance from the nucleus Circular or elliptical path No path: varied shape of region 2n2 electrons per orbit 2 electrons per orbital Quantum Numbers - Used to describe an electron in an atom - There are four quantum numbers: 1) 2) Principal Quantum Number (n) - Aka: Principal energy level - Tells you the energy of the electron in an atom - Positive number (1,2,3,…) - The smaller the n, the lower the energy - The size of an orbital also depents on n - Orbitals with the same n are said to belong to the same shell - Are sometimes designated by letters Letter K L M N… n 1 2 3 4… Secondary Quantum Number (l) - Aka: Sublevel - Distinguishes orbitals of a given n as having different shapes - An integer value from 0 to n – 1 - For a given n, the energy of an orbital increases with l - Orbitals of the same n but different l are said to belong to different subshells of a given shell Letter s p d f l 0 1 2 3 The subshells are split up into 4 different types s subshell – hold a max of 2 electrons p subshell – hold a max of 6 electrons d subshell – hold a max of 10 electrons f subshell – hold a max of 14 electrons - Shapes of the subshells s subshell – sphere p subshell – 3 dumbbells The shape of the subshells become harder to predict and the mathematical calculations are more complex as the principal quantum number increases s (l=0) p (l=1) m=0 m=0 s pz d (l=2) m=±1 px m=0 py dz2 f (l=3) m=±1 dxz m=±2 dyz dxy dx2-y2 m=0 fz3 m=±1 fxz2 fyz2 m=±2 fxyz m=±3 fz(x2-y2) fx(x2-3y2) fy(3x2-y2) n=1 n=2 n=3 n=4 n=5 n=6 n=7 ... ... ... ... ... ... ... ... ... ... ... ... ... ... ... ... ... ... ... ... ... ... ... ... ... ... ... ... ... ... ... ... ... ... 3) Magnetic Quantum Number (ml) - Distinguishes one orbital from another in the same sublevel Distinguishes orbitals given n and l → that is, given energy and shape but having a different orientation in space Integers from –l to +l Each orbital of a given subshell has the same energy Table 1. Permissible Values of Quantum Numbers for Atomic Orbitals n l ml * Subshell Notation Number of Orbitals in the Subshell 1 0 0 1s 1 2 0 0 2s 1 2 1 -1, 0, +1 2p 3 3 0 0 3s 1 3 1 -1, 0, +1 3p 3 3 2 -2, -1, 0, +1, +2 3d 5 4 0 0 4s 1 4 1 -1, 0, +1 4p 3 4 2 -2, -1, 0, +1, +2 4d 5 4 3 -3, -2, -1, 0, +1, +2, +3 4f 7 * Any one of the mi quantum numbers may be associated with the n and l quantum numbers on the same line. - NOTE: there are 2l + 1 orbitals in each subshell of quantum number l 4) Spin Quantum Number (ms) - Refers to the two possible orientations of the spin axis of an electron - Possible values are + ½ or – ½ Table 2. Key Experimental Work in Creating the Four Quantum Numbers Key Experimental work low-resolution line spectra Theoretical Explanation principal quantum number (n) high-resolution line spectra secondary quantum number (l) spectra in magnetic field magnetic quantum number (ml) ferro- and paramagnetism spin quantum number (ms) Quantum Theory All electrons in all atoms can be described by four quantum numbers Electron Configuration - Shows the order of filling of the orbitals 1s2 Energy level (n) Sublevel/subshell (l) Do examples: sulphur, iron, bismuth Number of electrons in that sublevel Shorthand of Electron Configurations - Write the symbol of the preceding noble gas in square brackets - Just the remaining electrons following that noble gas need to be included