Quantum Numbers Explained: A Chemistry Guide
advertisement
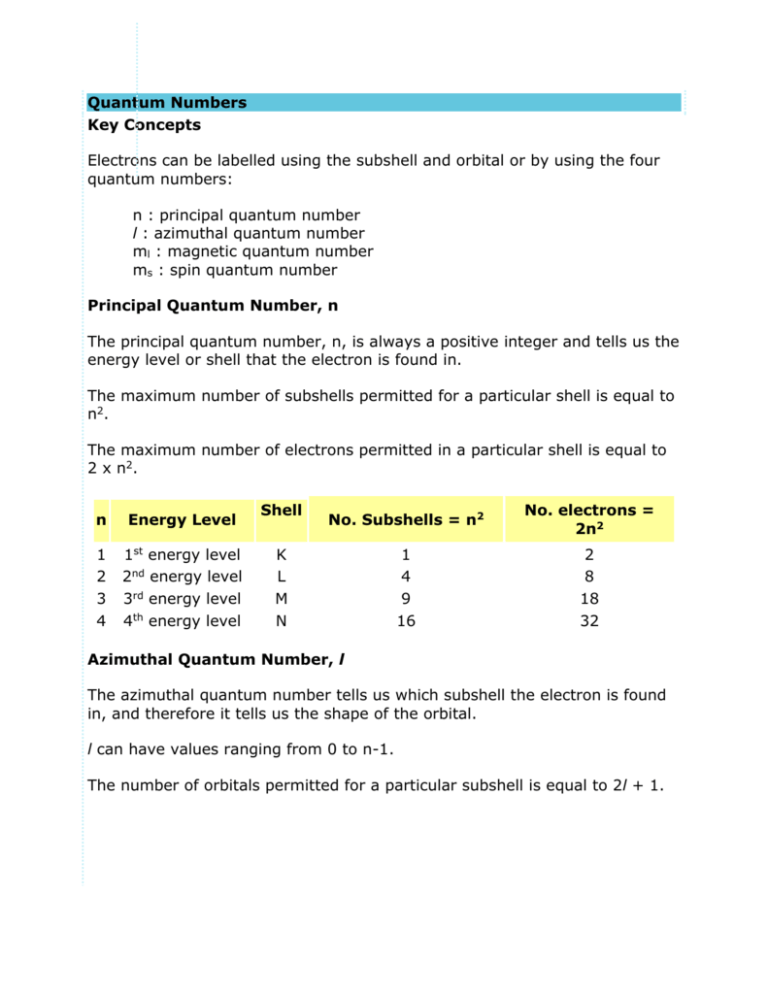
Quantum Numbers Key Concepts Electrons can be labelled using the subshell and orbital or by using the four quantum numbers: n : principal quantum number l : azimuthal quantum number ml : magnetic quantum number ms : spin quantum number Principal Quantum Number, n The principal quantum number, n, is always a positive integer and tells us the energy level or shell that the electron is found in. The maximum number of subshells permitted for a particular shell is equal to n2. The maximum number of electrons permitted in a particular shell is equal to 2 x n2. n Energy Level 1 2 3 4 1st energy level 2nd energy level 3rd energy level 4th energy level Shell No. Subshells = n2 No. electrons = 2n2 1 4 9 16 2 8 18 32 K L M N Azimuthal Quantum Number, l The azimuthal quantum number tells us which subshell the electron is found in, and therefore it tells us the shape of the orbital. l can have values ranging from 0 to n-1. The number of orbitals permitted for a particular subshell is equal to 2l + 1. Value of l subshell (orbital shape) No. orbitals = 2l + 1 0 1 2 3 s subshell p subshell d subshell f subshell 1 (1 x s orbitals) 3 (3 x p orbitals) 5 (5 x d orbitals) 7 (7 x f orbitals) Magnetic Quantum Number, ml The magnetic quantum number, ml, tells us the orientation of an orbital in space. ml can have values ranging from -l to +l. It is not always possible to associate a value of ml with a particular orbital. value of l subshell 0 1 2 3 s p d f values of ml possible orbitals 0 s -1, 0, 1 px, py, pz -2, -1, 0, 1, 2 dxy, dxz, dyz, dx2-y2, dz2 -3, -2, -1, 0, 1, 2, 3 Complex Spin Quantum Number, ms The spin quantum number, ms, tells us the spin of the electron. ms can have a value of +½ or -½. Example The argon atom has 18 electrons. The quantum numbers for each of the 18 electrons is shown below: electron n (shell) l (subshell) ml (possible orbital) ms 1 2 1 (K) 1 (K) 0 (s) 0 (s) 0 (1s) 0 (1s) -½ +½ 3 4 5 6 7 8 9 10 2 2 2 2 2 2 2 2 (L) (L) (L) (L) (L) (L) (L) (L) 0 0 1 1 1 1 1 1 (s) (s) (p) (p) (p) (p) (p) (p) 0 (2s) 0 (2s) -1 (2px) 0 (2py) +1 (2pz) -1 (2px) 0 (2py) +1 (2pz) -½ +½ -½ +½ -½ +½ -½ +½ 11 12 13 14 15 16 17 18 3 3 3 3 3 3 3 3 (M) (M) (M) (M) (M) (M) (M) (M) 0 0 1 1 1 1 1 1 (s) (s) (p) (p) (p) (p) (p) (p) 0 (3s) 0 (3s) -1 (3px) 0 (3py) 1 (3pz) -1 (3px) 0 (3py) 1 (3pz) -½ +½ -½ +½ -½ +½ -½ +½











