quiz 3 27 april 2009
advertisement

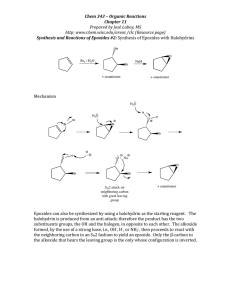
CH221 ORGANIC CHEMISTRY I QUIZ 3 27 APRIL 2009 Time allowed: 50 minutes Attempt all 35 questions on the grid answer sheet provided 1. Which of the following reagents adds to an alkene in an antiMarkovnikov fashion? A. B. C. D. 2. H2O/H+ HBr HCl [1] BH3; [2] H2O2/OH- In which of the following reactions will carbocation rearrangements occur? A. The addition of Br2 and H2O to a C Chalohydrin formation. B. The addition of Br2. C. The addition of H2O in the presence of acid. D. Reactions (The addition of Br2 and H2O to a C Chalohydrin formation) and (The addition of H2O in the presence of acid) will both show carbocation rearrangements. E. Reactions (The addition of Br2 and H2O to a C Chalohydrin formation), (The addition of Br2 ), and (The addition of H2O in the presence of acid) will all show carbocation rearrangements. 3. A compound M with molecular formula C8H12 and no triple bonds reacts with H2 to give a new compound having molecular formula C8H14. What can be said about compound M? A. Compound M has 3 rings. B. Compound M has 2 rings and 1 bond. C. Compound M has 1 ring and 2 bonds. D. Compound M has 3 bonds. E. It's impossible to say anything about the structure of compound M given this data. 4. 7. Which is not a product of this reaction? 8. What is the missing reagent in the reaction below? 9. From the list of possible carbocations, which one would not be likely to rearrange? 10. You are to synthesize 2-bromobutane from 1-butene. You must get the highest yield possible. From the list of reagents given below, which one would you use to obtain the greatest yield? A. B. C. D. E. Alcoholic KOH KOH and water t-Butyl alcohol and (CH3)3COK Ethanol and CH3CH2ONa Methanol and CH3ONa 11. Give the IUPAC name for the following compound. Predict the product(s). 12. What is the missing reagent in the reaction below? 5. Predict the product(s). 6. Predict the product(s). 13. What is the product in the reaction below? 1 14. Determine the product in the reaction below. 23. Which of the following alcohols undergoes dehydration most slowly on treatment with acid? 15. Determine the product in the reaction below. 16. Determine the major product in the reaction below. 17. Predict the product in the reaction below. 18. Determine the product in the reaction below. 24. For the compound drawn below, decide which statement(s) is (are) true. A. It is soluble in H2O. B. It is soluble in CHCl3. C. It contains a sp hybridized atom. D. The statements (It is soluble in H2O) and (It is soluble in CHCl3) are both true. E. None of the choices are correct. 25. Which of the following statements is (are) true about ring opening of epoxides with nucleophiles? 19. Determine the product in the reaction below. 20. Which of the following compounds has the highest boiling point? A. All nucleophiles ring open epoxides with backside attack. B. Ring opening of epoxides always follows an SN2 mechanism. C. Nucleophilic attack always occurs at the less substituted carbon atom. D. Statements (All nucleophiles ring open epoxides with backside attack) and (Ring opening of epoxides always follows an SN2 mechanism) are true. E. Statements (All nucleophiles ring open epoxides with backside attack), (Ring opening of epoxides always follows an SN2 mechanism), and (Nucleophilic attack always occurs at the less substituted carbon atom) are all true. 26. Determine the product in the reaction below. 21. Give the IUPAC name for the following compound. 27. Determine the major product in the reaction below. 22. Which of the following compounds has the highest boiling point? 2 28. What is the product? 36. A secondary alkyl halide reacts with a strong bulky base. The reaction will proceed via which of the following mechanism(s)? 29. What is the product? A. B. C. D. E. SN1 SN2 E1 E2 SN1 and E1 30. What is the likely mechanism of this reaction? 31. Which of the following halides is most reactive in an elimination reaction having first-order kinetics? 32. Which of the following statements is (are) true about an E2 elimination reaction? A. It is fastest with 3 halides. B. It exhibits second-order kinetics. C. A better leaving group should make a faster reaction. D. Both (It is fastest with 3 halides) and (It exhibits second order kinetics) are true. E. Statements (It is fastest with 3 halides), (It exhibits second order kinetics), and (A better leaving group should make a faster reaction) are all true. 33. Which of the labeled protons in compound A is most readily abstracted under conditions of E2 elimination? 34. Consider the following exothermic E2 reaction. What rate equation would be observed for this reaction? A. rate = k[CH3CH2CH2I] B. rate = k[CH3CH2CH2I][K+ -OC(CH3)3] C. rate = k[CH3CH2CH2I][K+ -OC(CH3)3]2 D. rate = k[CH3CH2CH2I]2[K+ -OC(CH3)3] E. There is not enough information given to determine the rate equation. 35. A tertiary alkyl halide reacts with a strong base. The reaction will proceed via which of the following mechanism(s)? A. B. C. D. E. SN1 SN2 E1 E2 SN1 and E1 3 CH221 QUIZ 2 ANSWER GRID Write in each box the answer you think corresponds to the correct answer to that question. Name…………………………………………….. Student Number…………………….…………… 1 2 3 D C B 4 5 6 7 8 B 9 10 C C 11 4-methylcyclohexene oxide 1,2-epoxy-4methylcyclohexane 12 13 14 15 19 20 ZnCl2, HCl Or SOCl2, pyridine 16 17 18 a>c>b 21 (E)-4-ethyl-2,5dimethyl-3octene 22 23 24 25 B C B A 26 27 28 29 30 SN2 31 C 32 33 E D 34 35 B 36 D D Total score 4