SCH 4U Name: Unit: Organic Chemistry Date: Aromatic
advertisement

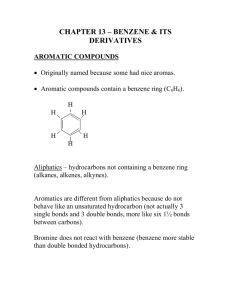
SCH 4U Name: ____________________ Unit: Organic Chemistry Date: ____________________ Aromatic Hydrocarbons Alkanes, alkenes and alkynes (whether straight chains, branched chains or cyclic structures) are all hydrocarbons. Together they are also classified as "aliphatic compounds". A fourth class of hydrocarbons exists. Instead of belonging to the aliphatic group, however, substances in this group are called "aromatic" compounds. They are easily recognized in the lab by their “aromatic” odour, which is often (but not always) sweet and enticing. Aromatic compounds are mostly derivatives of benzene (C6H6) (cyclohexatri– 1,3,5 – ene) The logical structure for benzene would be a hybrid (or combination) of the two resonance structures shown below. We will return to the idea of resonance structures in Unit 2… For now… …Suffice to say that it does not make chemical sense for the single and double bonds in benzene to shift back and forth. Here is some evidence to support the notion that benzene must have a very stable 6 carbon ring structure: 1) Benzene undergoes substitution reactions rather than addition reactions (ie. instead of adding reactants onto the ring directly by breaking π bonds between carbon atoms, the C – C “double bonds” in the ring are found to remain intact and hydrogen atoms outside the ring are removed and substituted for by the reactant(s)). (We’ll come back to this…The bottom line is that alkene and alkyne multiple bonds undergo addition not substitution reactions. Benzene “double bonds” do the opposite – so they must not be your standard multiple bonds...) 2) Because all of the carbon atoms in the benzene ring molecule react in the same way, all of the carbon – carbon bonds must be chemically equivalent to each other. This is a bit weird too because single and double bonds are typically different from one another in terms of bond length and chemical reactivity. 3) The carbon-carbon bonds in benzene are all of equivalent lengths. All are 139 pm long. This value is intermediate between the length of a carbon-carbon single bond (148 pm) and a carbon-carbon double bond (134 pm). These characteristics have led scientists to conclude that the p orbitals involved in creating the π bonds between the carbons overlap all around the benzene ring structure to give a continuous pi bond around the molecule. We indicate this arrangement by placing a circle inside the centre of the structural formula for the benzene ring. The condensed structural formula for benzene is a hexagon with a circle inside it. (see below) Right now, we do not know much about “p orbitals”. We will in the second unit. For now, it will suffice to just accept the condensed structural formula for benzene shown to the left. Alternatively, you will see it drawn as one of the resonance structures shown above with alternating double and single bonds. NOMENCLATURE FOR SIMPLE AROMATIC COMPOUNDS The following chart indicates that the IUPAC naming system for benzene derivatives is quite straight forward and follows established rules. The ortho-, meta- and para- designations indicated are still used today and you should be familiar with them. Similarly, you need to know the structures and names of toluene and xylene. Condensed Structural Formula IUPAC Name Methylbenzene 1, 2 – dimethylbenzene 1, 3 – dimethylbenzene 1, 4 – dimethylbenzene Common Name Toluene ortho-xylene meta-xylene para-xylene Condensed Structural Formula Br Br Br Br Br Br Br IUPAC Name Bromobenzene 1, 2 – dibromobenzene 1, 3 – dibromobenzene 1, 4 - dibromobenzene Common Name bromobenzene ortho - dibromobenzene meta- dibromobenzene para - dibromobenzene Other benzene derivatives you should be aware of: phenol aniline benzoic acid Note also that benzene can be an alkyl branch on a long or complicated carbon parent chain. When this happens, the branch name is phenyl. Practice 1. Draw structural formulas for each of the following aromatic compounds. a) 2,4,6-trichlorotoluene b) 1-fluoro-2-ethylbenzene c) 1,3,5-tribromobenzene d) para-ethyltoluene e) 1, 2 – diethylbenzene 2. Give the correct IUPAC name for each of the following compounds. (When benzene is named as an alkyl branch it has the name phenyl.) a) b) F More Practice Page 21 #7 and 8. c)