Reactions of Hydrocarbons
advertisement

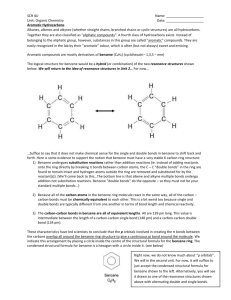
Section 1.3: Reactions of Hydrocarbons Reactions of Alkanes Combustion in the presence of O2 (Oxidation) Review Grade 11 Eg. Complete Combustion 2 C6H14 + 19 O2 12 CO2 + 14 H2O Eg. Incomplete Combustion C6H14 + 7 O2 2 CO2 + 7 H2O + C + 3 CO Substitution reactions with Halogens (Halogenation pg. 25) C-C bonds are hard to break but H atoms may be substituted for by halogens: F2, Cl2 or Br2 F2 will react without catalyst Cl2 and Br2 require heat or UV radiation to break apart the diatomic molecules Eg. General Equation Reactions of Alkenes and Alkynes C=C and CC are weak bonds that are easily broken making molecules that contain them more reactive than alkanes Addition Reactions double or triple bond is broken and replaced by atoms adding onto the molecule a) Halogenation F2, Cl2 or Br2 are all able to react with alkenes and alkynes without the use of catalysts Eg. General Equation b) Hydrogenation (with H2) requires heat, pressure, or a catalyst process used on oils to turn them into solid fats Eg. Margarine and Peanut Butter Eg. General Equation Markovnikov’s Rule When Alkene or Alkyne reacts with a hydrogen halide or water the H bonds to the Carbon with the most H already attached. “The Rich get Richer” c) Hydrohalogenation reactions occur at room temperature Eg. General Equation d) Hydration (with H2O) involve the addition of water (HOH) require a H2SO4 catalyst Eg. General Equation New symbols: Alkyl Groups Halogen Phenyl group R, R’, R’’, etc. (R, R-prime, R-double prime) (R = radical) X Ø New prefixes: bromoBrchloroClfluoroFhydroxyl-OH * organic and hydroxy - halides are polar (and therefore can be dissolved into water) nitro- -NO2 phenyl - - (benzene) Reactions with Aromatic Hydrocarbons Reactivity is intermediate between alkanes and alkenes. The Benzene structure is particularly stable. We show Benzene as having 3 C=C (double bonds) and 3 C-C (single bonds). This would suggest shorter, weaker C=C bonds alternating with longer, stronger C-C bonds. In actuality X-ray diffraction shows 6 equal length, equal strength bonds. The 18 valence electrons in Benzene are equally shared in a delocalized arrangement. – a unique bonding situation. Hence, the benzene ring does NOT undergo addition reactions that break apart double bonds – benzene rings DO NOT BREAK APART in hydrocarbon reactions. a) halogenation - substitution Rx of a halogen with cycloalkane requires heat or UV eg. halogenation – substitution Rx of a halogen with benzene (no addition) requires FeBr3 catalyst eg. Halogenation - substitution Rx of a halogen with cyclohexene occurs at room temperature (due to easy to break double bond) eg. Note that the benzene structure is not broken in the second reaction b) Nitration – substitution Rx of nitric acid with benzene requires H2SO4 catalyst eg. c) Alkylation – substitution Rx of an alkyl halide with benzene requires AlCl3 catalyst eg. BENZENE DOES NOT UNDERGO ADDITION REACTIONS