Signs of a Chemical Reaction
advertisement

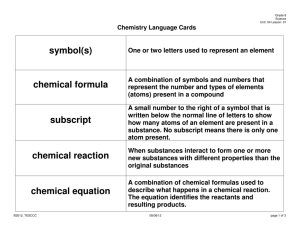
Name ______________________________________________Period_____ Understanding Chemical Reactions (Use with book pages 183-190) Changes in Matter 1. A _________________________ change does not produce new substances. 2. During a ___________________ change, new substances form. a. The starting substances and the substances __________________ have different physical and chemical ___________________________. b. A chemical reaction is a process in which ____________________ of one or more substances rearrange for form one or more new ____________. Signs of a Chemical Reaction 1. Sometimes, changes in ______________________ properties, such as color or odor, indicate a chemical reaction. a. Formation of bubbles of a ____________________ can also be a sign of a chemical reaction. b. A solid formed when two liquids are mixed is called a _______________ and can be evidence of a chemical reaction. 2. A change in ____________________ is another sign that a chemical reaction has occurred. a. _______________________ energy is absorbed or released during a chemical reaction and is evidenced by warming or cooling. b. ______________________ energy might also be released during a chemical reaction. 3. The only way to be certain a chemical reaction has taken place is to compare the ____________________ of the substances before and after the change. What happens in a chemical reaction? 1. During a chemical reaction, _________________________of elements or compounds ___________________ and form new elements or compounds. 2. Atoms rearrange when chemical ________________between atoms break. Chemical Equations 1. A ____________________________is a description of a reaction using element _______________________ and chemical formulas. 2. Element symbols represent ____________________________________. a. Symbols of elements are shown on the __________________________. b. When an element exists as a _______________________ molecule, the element symbol is followed by the subscript 2. 3. Chemical formulas represent __________________________. a. A chemical formula contains ____________________ symbols and __________________________ to describe the makeup of a compound. b. When chemical formulas differ, the represent different _____________. 4. A chemical equation includes the substances that _____________________ and the substances _______________________________. a. ________________________ are the starting substances in a reaction. b. _______________________ are the substances produced by a reaction. c. In a chemical equation, the ____________________ are written to the left of an arrow, and the ______________________ are written to the right of the arrow. d. Two or more reactants are separated by ____________________ signs, as are any two or more products. Conservation of Mass 1. The law of _____________________________ states that the total mass of the reactants before a reaction is the same as the total mass of the products after the chemical reaction. 2. Mass is conserved in a chemical reaction because ___________________ are conserved. 3. To show that mass is conserved, a chemical equation must show that the number of each type of __________________________________ must be ________________________, or the same on both sides of the equation. a. Chemical equations are balanced by adding _______________________. b. A coefficient is a _________________________ placed in front of an element symbol or a chemical formula in an equation. c. In the formula 2H2O, the 2 in front off H2O is a coefficient. This means there are __________ ____________________ of water in the reaction. d. Only ____________________________ can be changed when balancing an equation. Explain this with the water example 1 – Write the unbalanced equation 2- Count atoms of each element in the reactants and the products. 3 – Add coefficients to balance atoms