Chapter 6: Matter Waves
advertisement
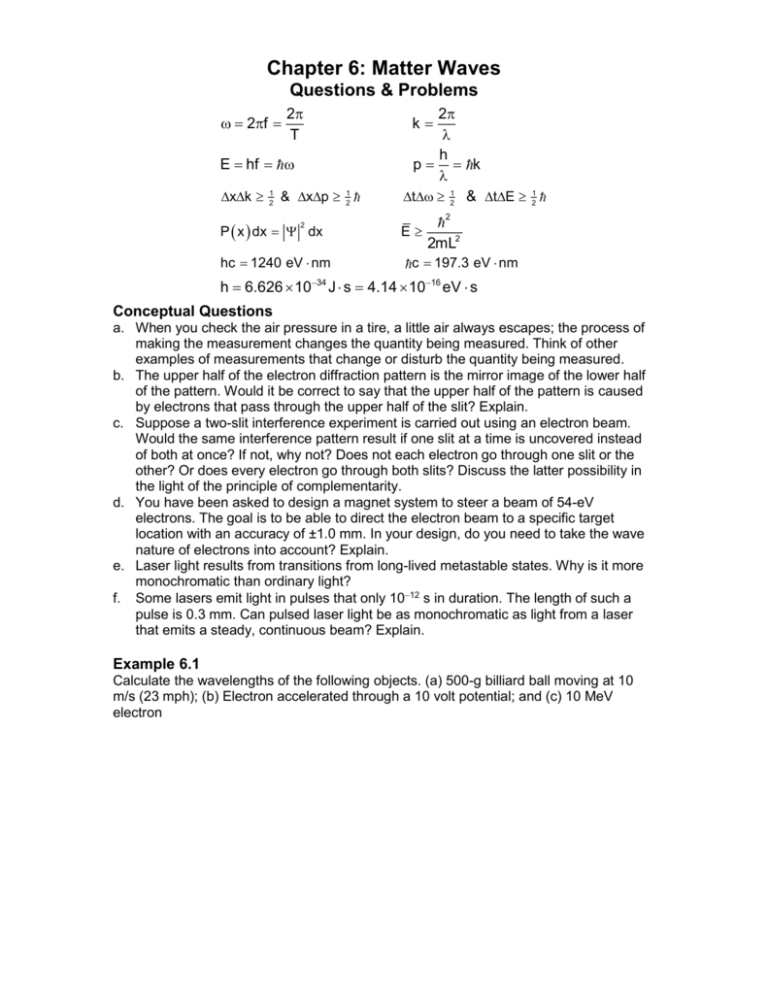
Chapter 6: Matter Waves Questions & Problems 2f 2 T E hf xk 1 2 & xp P x dx dx E hc 1240 eV nm 34 1 2 1 2 2 2 h 6.626 10 2 h p k t 21 & tE k 2mL2 c 197.3 eV nm J s 4.14 10 16 eV s Conceptual Questions a. When you check the air pressure in a tire, a little air always escapes; the process of making the measurement changes the quantity being measured. Think of other examples of measurements that change or disturb the quantity being measured. b. The upper half of the electron diffraction pattern is the mirror image of the lower half of the pattern. Would it be correct to say that the upper half of the pattern is caused by electrons that pass through the upper half of the slit? Explain. c. Suppose a two-slit interference experiment is carried out using an electron beam. Would the same interference pattern result if one slit at a time is uncovered instead of both at once? If not, why not? Does not each electron go through one slit or the other? Or does every electron go through both slits? Discuss the latter possibility in the light of the principle of complementarity. d. You have been asked to design a magnet system to steer a beam of 54-eV electrons. The goal is to be able to direct the electron beam to a specific target location with an accuracy of ±1.0 mm. In your design, do you need to take the wave nature of electrons into account? Explain. e. Laser light results from transitions from long-lived metastable states. Why is it more monochromatic than ordinary light? f. Some lasers emit light in pulses that only 1012 s in duration. The length of such a pulse is 0.3 mm. Can pulsed laser light be as monochromatic as light from a laser that emits a steady, continuous beam? Explain. Example 6.1 Calculate the wavelengths of the following objects. (a) 500-g billiard ball moving at 10 m/s (23 mph); (b) Electron accelerated through a 10 volt potential; and (c) 10 MeV electron Example 6.2 a. The position x of a 0.01-g pellet has been carefully measured and is known within ±0.5 m. According to the uncertainty principle what are the minimum uncertainties in its momentum and velocity, consistent with our knowledge of x? b. An electron is known to be somewhere in an interval of total width a 0.1 nm (the size of a small atom). What is the minimum uncertainty in its velocity, consistent with this knowledge? Example 6.3 Many excited states of atoms are unstable and decay by emission of a photon in a time of order t 10-8 s. What is the minimum uncertainty in the energy of such an atomic state? Example 6.4 Suppose that a particle of mass m is constrained to move in a one-dimensional space between two infinitely high barriers located A apart. a. Compute the minimum energy of an electron for A = 1010 m and A = 102 m. Interpret the results. b. Calculate the minimum energy for a 100-mg bead moving on a thin wire between two stops located 2 cm apart. Interpret the results. Example 6.5 It is possible for some fundamental particles to “violate” conservation of energy by creating and quickly by reabsorbing another particle. For example, a proton can emit a + according to p n where n represents a neutron. The + has a mass of 140 MeV/c2. The reabsorption must occur within a time t consistent with the uncertainty principle. (a) By how much E is energy conservation violated? (Ignore KE) (b) For how long t can the + exist? (c) Assuming that the is moving at nearly the speed of light, how far from the nucleus could it get in the time t?











