Untitled document - British Renal Society
advertisement

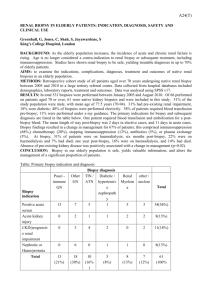
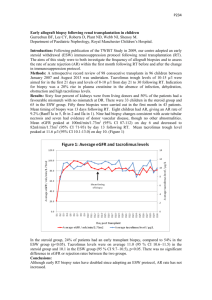
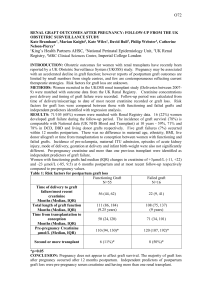
P258 DETERIORATING RENAL FUNCTION IN LONG-TERM RENAL ALLOGRAFTS: DOES ALLOGRAFT BIOPSY PROVIDE EFFECTIVE SOLUTIONS EVEN AT A LATE STAGE? P. Nagaraja¹, D.O. Rees¹, R. Shrivastava¹, S. Howarth², C. Parker¹, V.S. Aithal¹ ¹Renal Unit and ²Department of Pathology, Morriston Hospital, Swansea. Background and aims: Preservation of graft function in long-term renal allografts has remained a challenge despite sustained improvements in 1-year graft survival. In this retrospective review of kidney transplant biopsies, we aimed to study the utility of allograft biopsy in the management of long-term renal transplants. We also studied the correlation between the clinical markers of graft dysfunction, histopathological findings and graft outcomes in this setting. Methods: All kidney transplant biopsies (n=38) undertaken in a single non-transplanting centre between 2008 and 2012 were reviewed. The average annual number of transplant patients under follow-up was 180. Acute graft dysfunction (AD) was defined as a sustained 25% rise in serum creatinine. All biopsies were analysed by a renal pathologist. Logistic regression was used to identify factors at the time of the biopsy which predicted death-censored graft loss (DCGL). Results: Median post-transplant follow-up time was 124 months (range 12-309). Indications for biopsy were AD (n=13) and chronic graft dysfunction and/or significant proteinuria (CD, n=25). Median time from transplantation to biopsy was 78 months (range 9-293 months). At the time of the biopsy, median S. creatinine level in patients with AD was 224 μmol/l (122-322) and in those with CD was 213 μmol/l (113-596). Acute cellular rejection (AR) was seen in 7/13 (54%) patients with AD. Recurrent glomerulonephritis (GN), ischaemic changes and non-specific glomerulosclerosis formed the next common diagnoses in patients with AD. In patients with CD, transplant glomerulopathy (TG) and recurrent GN were the most prevalent diagnoses (75% overall). BK virus nephropathy and CNI toxicity featured in both groups. The biopsy findings led to a change in the immunosuppression regimen in 14/38 patients (37%). The overall DCGL rate was 50%. Patients with AR were no more likely to lose their grafts than those without AR (43% vs. 52%, p=0.6). Two patients each with BK virus nephropathy and CNI toxicity responded well to immunosuppression reduction and stabilised graft function. Pre-biopsy serum creatinine (OR 1.2 for every 10 μmol/l rise, p=0.003) and presence of proteinuria (OR 12.9, p=0.04) predicted graft loss. On the other hand, neither the indication (AD or CD) nor the biopsy diagnosis (AR, TG or GN) was associated with graft loss. Median time to DCGL was 8 months from the time of biopsy (range 0-44). There were no complications resulting from the biopsies. Conclusions: In this single-centre retrospective review, we found that allograft biopsy undertaken in long standing renal transplants was safe but contributed to a change in immunosuppression in only a minority of patients. The biopsy findings permitted aggressive reductions in immunosuppression in the small sub-group with BKVN and CNI toxicity and the impact on graft stabilisation was most significant in this sub-group. Pre-biopsy serum creatinine and the presence of proteinuria, but not histopathological diagnoses, predicted graft loss.