Stoichiometry WS 9 Percent Yield - Sayers-ONeill
advertisement

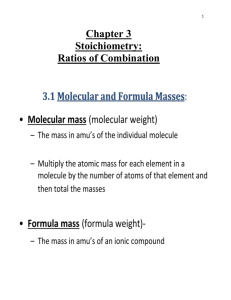
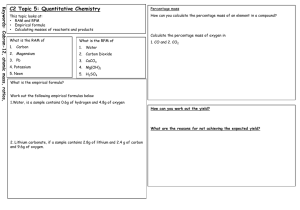
Stoichiometry Unit Worksheet #9: Percent Yield percentage yield = actual amount of product X 100 (Calculation is just like calculating your % on a quiz) expected amount of product Example 1: A student conducts a reaction that produces 2.755 grams of copper. Mathematically he determines that 3.150 grams of copper should have been produced. Calculate the student's % yield. Answer: actual amount of product X 100 = 2.755 g X100 = 87.5% expected amount of product 3.150 g 1. In the reaction shown below, a student produces 22 grams of water. Mathematically he determines that 29 grams of water should have been produced. Calculate the student's percentage yield. 2H2 + O2 synthesis 2. The actual amount of MgO synthesized in the reaction shown below was 0.77 g. If the theoretical amount of MgO was calculated to be 1.30 g, what is the percent yield of the reaction? 2Mg + O2 synthesis 3. A chemist starts with 11.2 g of KClO3 and calculates that it should be possible to make 7.6 g of KCl with that much KClO3. After having conducted the reaction, she finds that she recovered only 6.8 g of KCl. What is her percent yield? 2KClO3 Decomposition (oxygen is one of the 2 products) 4. You have 6.2 g of NH3 in the cabinet. You do a short calculation and figure out that this much NH3 will yield 0.92 g of H2 in the reaction shown below. You conduct the reaction and find that you actually get 0.90 g of H2. What is your percent yield? 2NH3 decomposition Stoichiometry Unit Worksheet #9: Percent Yield percentage yield = actual amount of product X 100 (Calculation is just like calculating your % on a quiz) expected amount of product Example 1: A student conducts a reaction that produces 2.755 grams of copper. Mathematically he determines that 3.150 grams of copper should have been produced. Calculate the student's % yield. Answer: actual amount of product X 100 = 2.755 g X100 = 87.5% expected amount of product 3.150 g 1. In the reaction shown below, a student produces 22 grams of water. Mathematically he determines that 29 grams of water should have been produced. Calculate the student's percentage yield. 2H2 + O2 synthesis 2. The actual amount of MgO synthesized in the reaction shown below was 0.77 g. If the theoretical amount of MgO was calculated to be 1.30 g, what is the percent yield of the reaction? 2Mg + O2 synthesis 3. A chemist starts with 11.2 g of KClO3 and calculates that it should be possible to make 7.6 g of KCl with that much KClO3. After having conducted the reaction, she finds that she recovered only 6.8 g of KCl. What is her percent yield? 2KClO3 Decomposition (oxygen is one of the 2 products) 4. You have 6.2 g of NH3 in the cabinet. You do a short calculation and figure out that this much NH3 will yield 0.92 g of H2 in the reaction shown below. You conduct the reaction and find that you actually get 0.90 g of H2. What is your percent yield? 2NH3 decomposition