C2_Chemistry_Summary_Topic_6
advertisement
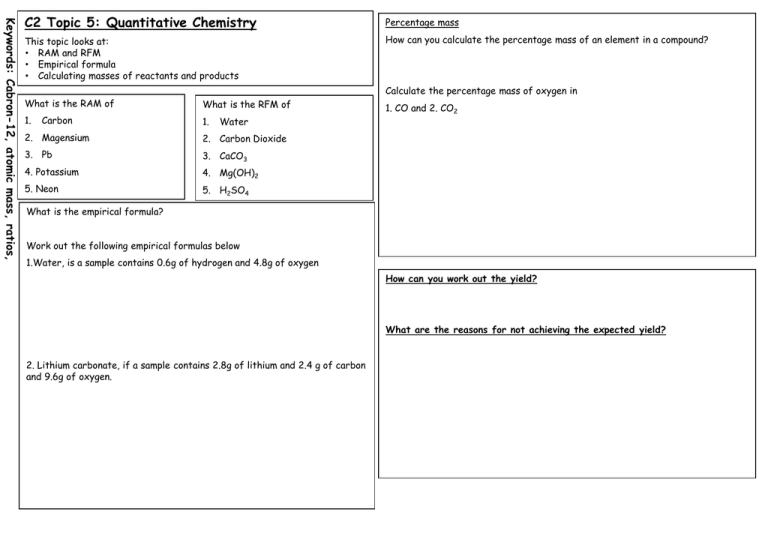
Keywords: Cabron-12, atomic mass, ratios, C2 Topic 5: Quantitative Chemistry Percentage mass This topic looks at: • RAM and RFM • Empirical formula • Calculating masses of reactants and products How can you calculate the percentage mass of an element in a compound? Calculate the percentage mass of oxygen in What is the RAM of What is the RFM of 1. 1. Carbon Water 2. Magensium 2. Carbon Dioxide 3. Pb 3. CaCO3 4. Potassium 4. Mg(OH)2 5. Neon 5. H2SO4 1. CO and 2. CO2 What is the empirical formula? Work out the following empirical formulas below 1.Water, is a sample contains 0.6g of hydrogen and 4.8g of oxygen How can you work out the yield? What are the reasons for not achieving the expected yield? 2. Lithium carbonate, if a sample contains 2.8g of lithium and 2.4 g of carbon and 9.6g of oxygen. Keywords: Cabron-12, atomic mass, ratios, C2 Topic 5: Quantitative Chemistry Calculating Reactants and Products This topic looks at: • RAM and RFM • Empirical formula • Calculating masses of reactants and products 1. a. What is the theoretical yield that could be obtained from 256g of sulfur dioxide. 2SO2 + O2 2SO3 Name 3 products made in the petrochemical industry 1. 2. 3. b. The actual yield of sulfur trioxide produced is 202g. Calculate the percentage yield. Why is it expensive to dispose of waste products? 2. a. Iron is extracted from iron oxide by reducing with carbon Fe2O3 + 3CO 2Fe + 3CO2 Using ideas about waste products explain why people would object to a proposal to build a new factory to manufacture plastics near to their home? Calculate the theoretical yield of iron that could be obtained from 320 g of iron oxide. b. The actual yield of iron produced is 89.6g. Calculate the percentage yield.