Lab * Determining the Latent Heat of Fusion for Ice
advertisement
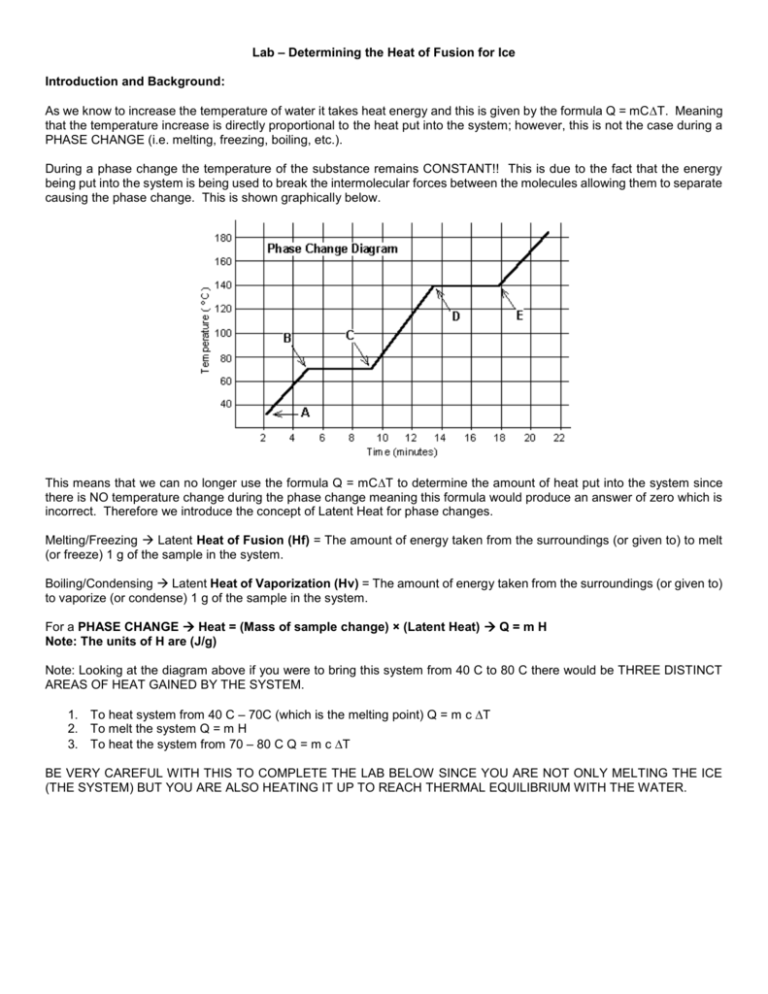
Lab – Determining the Heat of Fusion for Ice Introduction and Background: As we know to increase the temperature of water it takes heat energy and this is given by the formula Q = mCT. Meaning that the temperature increase is directly proportional to the heat put into the system; however, this is not the case during a PHASE CHANGE (i.e. melting, freezing, boiling, etc.). During a phase change the temperature of the substance remains CONSTANT!! This is due to the fact that the energy being put into the system is being used to break the intermolecular forces between the molecules allowing them to separate causing the phase change. This is shown graphically below. This means that we can no longer use the formula Q = mCT to determine the amount of heat put into the system since there is NO temperature change during the phase change meaning this formula would produce an answer of zero which is incorrect. Therefore we introduce the concept of Latent Heat for phase changes. Melting/Freezing Latent Heat of Fusion (Hf) = The amount of energy taken from the surroundings (or given to) to melt (or freeze) 1 g of the sample in the system. Boiling/Condensing Latent Heat of Vaporization (Hv) = The amount of energy taken from the surroundings (or given to) to vaporize (or condense) 1 g of the sample in the system. For a PHASE CHANGE Heat = (Mass of sample change) × (Latent Heat) Q = m H Note: The units of H are (J/g) Note: Looking at the diagram above if you were to bring this system from 40 C to 80 C there would be THREE DISTINCT AREAS OF HEAT GAINED BY THE SYSTEM. 1. To heat system from 40 C – 70C (which is the melting point) Q = m c T 2. To melt the system Q = m H 3. To heat the system from 70 – 80 C Q = m c T BE VERY CAREFUL WITH THIS TO COMPLETE THE LAB BELOW SINCE YOU ARE NOT ONLY MELTING THE ICE (THE SYSTEM) BUT YOU ARE ALSO HEATING IT UP TO REACH THERMAL EQUILIBRIUM WITH THE WATER. Lab – Determining the Latent Heat of Fusion for Ice Materials: Calorimeter (Styrofoam cup in a 400 mL beaker with a cardboard cup lid), Lab Quest with temperature probe, Graduated cylinder, ice Method: 1) Obtain a Styrofoam cup, cardboard lid, Lab quest with temperature probe 2) Measure the mass of an empty Styrofoam cup. Record, 3) Using the graduated cylinder add 70 mL of hot tap water to the Calorimeter. Measure the mass of the Styrofoam cup and water. Record. 4) Take the initial temperature of the water. Record. 5) Obtain a small amount of free melting ice. 6) Place the ice into the calorimeter. DO NOT touch the ice with your fingers. Dry the ice so that no melt water is added to the Styrofoam cup. Allow the ice to melt and record the final temperature of the mixture. 7) Measure the mass of water, melted ice and Styrofoam cup. Record. Use this value to determine the amount of ice that was melted. Record. 8) Repeat this experiment 3 more times using a different amount of ice cubes. 9) Use your results to determine the Latent Heat of Fusion of Ice. a. Calculate the energy (Q) released from the hot water. b. Calculate the energy (Q) absorbed by the melt water as it came to the final temp Energy released by hot water = Energy absorbed to melt ice + Energy absorbed to increase temp of melt water (m × ΔT × c) = (m × Hf) + (m ×ΔT × c) Trial 1 Trial 2 Trial 3 Mass of empty cup (do each time) Mass of cup and hot water Mass of hot water only Initial Temp of hot water Final Temp of hot water w/ melted ice Change in temp of hot water Initial temp of melted ice-water (Known, not measured!) Change in temp of melted ice water Mass of cup, hot water & melted ice water Mass of ice/melted water Specific Heat of water (known from notes) *****Solve for Hf above for each of the three trials on a separate piece of paper.