Heat of Fusion of Ice
advertisement

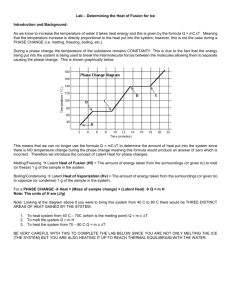
Lab: Determination of the Heat of Fusion of Ice Purpose: In this lab, you will determine experimentally the heat of fusion of ice, ΔHfus. This is the amount of heat energy required to melt 1.00 g ice at its melting point of 0.00 oC. Procedure 1. Determine the mass of a clean, dry styrofoam cup. 2. Add enough tap water to the styrofoam cup to about 1/2 full. 3. Determine the mass of the styrofoam cup and the water. Subtract the mass of the cup from the mass of the cup and water. Record the mass of the water alone in the Data Table as M1. Remove the styrofoam cup with water from the balance. 4. Immediately measure the temperature of the water in the styrofoam cup as accurately as possible and record this initial temperature as T1 in the DataTable. 5. IMMEDIATELY add 2-3 ice cubes to the water in the Styrofoam cup and begin stirring gently with the thermometer. Stir continuously! As the last of the ice appears about to melt, add another ice cube. The cup should contain ice at all times. 6. Continue stirring and adding ice as needed until the temperature of the water/ice mixture no longer decreases. It should be less than 4⁰C. Record this final temperature as T2 in the Data Table. 7. Using tongs, carefully remove the unmelted ice – allow as much water as possible to drip from the unmelted ice into the styrofoam cup. Place the unmelted ice into the sink. 8. Mass the water that is now in the styrofoam cup. Subtract the mass of the empty dry cup from the mass of the cup and water. Record this final mass of the water alone as M2 in the Data Table. 9. Repeat Steps 1 – 8 for one more trial. 1 DATA TABLE Mass of clean, dry styrofoam cup (g) Original Mass of Water (g) (M1) Mass Final Mass of of Water (g) Melted Ice (M2) (g) Trial # Initial Water Temp (oC) Final Water Temp (oC) Change in Temp (oC) (T1) (T2) (T) (M2 M1) 1 2 Calculations (Do these for both trials) 1. How much heat was lost by the water? 2. How much heat was gained by the ice? How do you know this? (No calculation required!) 3. Calculate the heat of fusion of ice in J/g. 4. Calculate the heat of fusion of ice in kJ/mol. 5. Calculate your percent error using the accepted value for the molar heat of fusion (6.01 kJ/mol). Post Lab Questions 1. Using evidence from this lab, explain how calorimetry is a demonstration of the law of conservation of energy. 2. a) Is the process of melting endothermic or exothermic? b) State evidence from this lab to support your answer. 3. How much heat is required to melt 10.5 g of ice at 0⁰C? Conclusion 2