File
advertisement

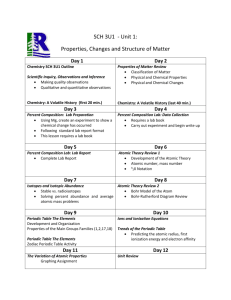
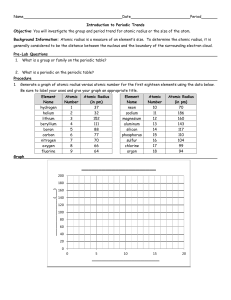
Using Excel to Graph Periodic Trends Objective: The student will graph and explain the trends found on the Periodic Table. Problem: Are certain properties of elements periodic with respect to their atomic numbers? Materials: graph paper, computer with Microsoft Excel, possibly colored pencils. Procedures: In this activity you will be making 5 graphs. The first will be done on graph paper (hand drawn) while the rest will be done using Excel. Each graph will use the data found in the section of the Periodic Table that is found below. Trendlines are not necessary for these graphs. Graph 1a: Homework For elements 3-20, use the data in the abridged periodic table to graph atomic radius as a function of atomic number. Atomic number is the independent variable for all graphs. Be sure to properly space the graph and label all aspects accordingly. This one is to be done on graph paper (provided to you in class). Excel Graph1b. Re-create your hand drawn graph (1a) using Excel. 1. Open an Excel Workbook 2. In Cell A1, type Element Symbol a. If the column is too narrow, put your cursor on the line between A and B the cursor will turn into a down arrow. Click once and the column will extend to width of your text. 3. In cell B1 type Atomic Number 4. In cell C1, type Atomic Radius (pm) [pm stands for picometers] 5. Use the data on the abridged periodic table you have been given a. Enter element symbols for elements 3-20 in column A b. Enter atomic numbers 3 -20 in the column B c. Enter the corresponding atomic radius in column C 6. To create a graph of your data: a. Highlight the columns headed Atomic Number and Atomic Radius b. Choose Insert / Charts/Scatter with Markers only c. Voila! A graph will appear. d. Click on your graph. e. Click Design /Chart Layout / Choose Layout 1. This will enable you to include a title and axis labels. Add an appropriate title and include axis labels with units where necessary. Click on the current title and type over it. f. Double click on the tab that says Sheet 1 at the bottom of the page. Rename the page Atomic Radius. Excel Graph 2. Atomic radius by Group 7. Open a new page. Click on Sheet 2. 8. In Cell A1, type Element Symbol. 9. In cell B1 type Atomic Number 10. In cell C1, type Atomic Radius Group 1 (pm) 11. In cell D1 , type Atomic Radius Group 2 (pm) 12. Use the data on the abridged periodic table you have been given for the laboratory… a. Enter element symbols for elements Groups 1 in column A followed by the element symbols for Group 2 b. Enter atomic numbers that correspond to the element symbols in column B c. Enter the corresponding atomic radius for Group 1 in column C and for Group 2 in column D. You will have blanks in both columns. d. Highlight columns B, C and D. e. Create a graph in Excel , as above) and rename the sheet Atomic Radius by Group. 13. To create a graph of your data: a. Highlight the columns headed Atomic Number and Atomic Radius b. Choose Insert / Charts/Scatter with Markers only c. Voila! A graph will appear. d. Click on your graph. e. Click Design /Chart Layout / Choose Layout 1. This will enable you to include a title and axis labels. Add an appropriate title and include axis labels with units where necessary. Click on the current title and type over it. f. Double click on the tab that says Sheet 1 at the bottom of the page. Rename the page Atomic Radius by Group. Excel Graph 3. Ionization Energy Using the same technique and data from the abridged periodic table create a new page and a graph for Excel Graph 3. Ionization Energy Excel Graph 4. Ionization Energy by Group Using the same technique as Graph 2 and data from the abridged periodic table create a new page and a graph for Excel Graph 4. Ionization Energy by Group. Use your graphs and your textbook to answer the following questions in your own words. 1. What happens to the atomic radius as the atomic number increases across a period? Down a group? Explain. 2. What happens to the energy needed to remove an electron [ionization energy] as the atomic number increases across a period? Down a group? Explain. 3. Are these properties of the elements periodic functions of their atomic number? Why or why not? You can cut and paste your answers for the questions above into a textbox on your final worksheet. Save your file as << lastnamefirstinitial >>and email it to me. Hints: Save your work often. Up to 10 bonus points for exceptional formatting. Use your extra time to improve your charts and graphs. Do this on a copy of your Excel Workbook in case you make any errors. Send your completed file to me at sara.anderson@kenton.kyschools.us Source: Goodsell, J. (n.d.). Using Excel to Graph Periodic Trends. Retrieved October 19, 2015, from https://www.fsd1.org/schools/wilson/jgoodsell/Documents/chemunit3_periodicity/Exce lLab.pdf