Unit 9 Solutions Notes
advertisement

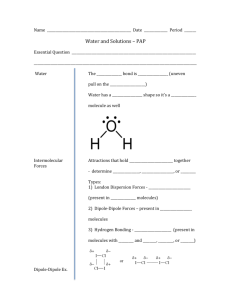
Unit 9: Properties of Solutions, Kinetics, and Equilibrium Vocabulary Solution – a mixture Solvent – substance being Solute – substance doing the Aqueous – solutions with as the solvent Alloy – a solid solution of (like bronze or steel) Solvation – to become surrounded by molecules Hydration – to become surrounded by molecules Solubility – amount of substance that can in a given amount of Saturated – containing amount of dissolved Unsaturated – containing than the maximum Supersaturated – containing than the maximum; often prepared at a Precipitate – undissolved that settles at bottom of solution Miscible – two that will in each other Immiscible – two that will not in each other Concentration – amount of in a given amount of Dilute - to Concentrated - ; substance that ratio of ratio of is dissolved in at a given ; additional solute will settle temperature to How fast a solute will dissolve depends on 3 factors: 1. Stirring (agitation) 2. Temperature 3. Particle size How much of a solute will dissolve depends on and also nature of solvent and solute. The solubility of solids tends to with temperature The solubility of gases tends to with temperature Golden rule of solubility: dissolves . This means that solutes will dissolve in solvents and solutes will dissolve in solvents. A mixture of polar and nonpolar components will not dissolve in each other. Temperature/Solubility Graph a) What is the solubility of KClO3 at 50°C in 100 g of water? b) Which salt is most soluble at 50°C? c) How much KCl could be dissolved in 300 g of water at 80°C? d) At 60°C, 41 g of NaCl are dissolved in 100 g of water. Is this solution saturated, unsaturated, or supersaturated? e) A saturated solution of NaNO3 is prepared at 50°C and then cooled to 0°C. How many grams of NaNO3 will precipitate out? Molarity Formula: Examples a) What is the molarity of a KCl solution with a volume of 400. mL that contains 85.0 g of KCl? b) Find the number of grams of AgNO3 needed to make 375 mL of a 0.750 M solution. c) How many mL of a 0.500 M solution of CuSO4 are needed to react with Al to provide 11.0 g of Cu? 3 CuSO4 + 2 Al 3 Cu + Al2(SO4)3 Dilution To perform a dilution, additional pure During a dilution, (or remain constant. is added to a prepared solution. ) and change, but Formula: Examples a) How many mL of 8.0 M HCl are needed to make 500. mL of 0.50 M HCl? b) Water is added to 50.0 mL of a 1.00 M NaCl solution until the new volume is 75.0 mL. What is the new molarity? c) 100.0 mL of water are added to 25.0 mL of 3.59 M HNO3. What is the new molarity? Colligative Properties Properties that depend on the number of moles of particles present in a solution but not on the identity of those particles. Ionic compounds dissociate when they dissolve to produce more particles. Molecular compounds (like glucose, C6H12O6) do not dissociate. NaCl K2SO4 3 Examples of Colligative Properties: 1) Vapor pressure lowering Nonvolatile (not easily evaporated) solutes lower the vapor pressure of solutions by taking up space at the surface of the solution 2) Boiling point elevation Recall that boiling occurs when pressure equals pressure Since the addition of a solute lowers the vapor pressure of the solution, adding a solute will the boiling point 3) Freezing point depression Particles of solute disrupt orderly pattern of atoms/ions in a solid, so freezing point is Net Ionic Equations and Solubility General Rules for Solubility of Ionic Compounds in Water 1. Most nitrate (NO3-) salts are soluble. 2. Most salts of Na+, K+, and NH4+ are soluble. 3. Most chloride salts are soluble. Exceptions are AgCl, PbCl2, and Hg2Cl2. 4. Most sulfate salts are soluble. Exceptions are BaSO4, PbSO4, and CaSO4. 5. Most hydroxide compounds are insoluble. Exceptions are NaOH and KOH. 6. Most sulfide (S2-), carbonate (CO32-), and phosphate (PO43-) salts are insoluble. Writing net ionic equations involves first writing a double displacement reaction and then predicting whether the products are soluble or not based on the above rules. Spectator Ions: ions that remain soluble as a reactant and a product. Example 1 Write the complete balanced equation, full ionic equation, and net ionic equation for the reaction of potassium iodide with lead(II) nitrate. Example 2 Write the complete balanced equation, full ionic equation, and net ionic equation for the reaction of silver nitrate with potassium sulfate. Example 3 Write the complete balanced equation, full ionic equation, and net ionic equation for the reaction of sodium hydroxide with sulfuric acid. NOTE: If all products are soluble, then NO REACTION OCCURS. For example, sodium chloride reacts with ammonium nitrate. Water and Aqueous Solutions Notes I. The Water Molecule Water is highly ; this means that the oxygen atom has a slightly charge and the hydrogens have a slightly charge. Water has a bond angle of Most of the unique properties of water are due to the fact that it forms II. Surface Properties of Water Water molecules at the surface can form hydrogen bonds with below them but not with molecules of above them; this uneven attraction forms a “skin” on the surface of water When forming droplets, two forces compete for the water molecules: pulls the drops into spheres while flattens the drops Soaps and detergents act as ; they weaken the surface tension of water, causing droplets to spread out Hydrogen bonding also acts to the vapor pressure of water. Water molecules at the surface cannot evaporate because they are held tightly by the water molecules below them. III. Evaporation of Water Water has a heat capacity and so heats up Water a lot of heat when it evaporates (so sweating cools you off) Water a lot of heat when it condenses (which is the reason for the severity of steam burns) Water has a molar mass, which would predict that it would be a or low-boiling liquid at room temperature; because of hydrogen bonds, water remains a at room temperature IV. Ice Above 4°C, the density of water will as it cools Below 4°C, the density of water will as it cools This means that ice will on liquid water V. Aqueous Solutions Aqueous solutions are ones that have as the solvent What types of compounds will dissolve in water? What types of compounds will not dissolve in water? VI. Hydration Hydration means to be surrounded by molecules Some ionic compounds will not dissolve because the attraction between ions in crystals are stronger than those between the ions and water Nonpolar molecules will not dissolve in water because water is more attracted to other water molecules than to nonpolar solute VII. Electrolytes and Nonelectrolytes Electrolytes are substances that can conduct electricity when or in state All compounds are electrolytes Most compounds are nonelectrolytes Strong electrolytes are good conductors because nearly all of the solute dissociates; weak electrolytes are poor conductors because most of the solute does not dissociate VIII. Suspensions and Colloids Suspensions are mixtures that upon standing The particles in suspensions are than those in solutions Colloids have particles of intermediate size; they do not settle, but are not true solutions o Tyndall Effect: Colloids will scatter o Brownian motion: The chaotic movement of colloidal particles Emulsions are colloids made of REACTION RATES (CHEMICAL KINETICS) over in order to Reaction rate: a change in Collision theory: atoms must reaction if: (1) (2) The minimum amount of energy required for a reaction to occur is called Reaction Coordinate Diagram Fast vs. Spontaneous A spontaneous reaction is one that will occur under indicated conditions . Collisions will only result in a Rate addresses how fast it will occur. This to a large extent depends on reaction with a large activation energy will only occur very of energy to overcome activation energy barrier. . A , or will not occur without an initial input Factors Affecting Reaction Rate (1) Temperature As temperature , reaction rate As temperature increases, more reactants have sufficient . Also, more kinetic energy leads to more collisions, but this is a much smaller effect (2) Concentration Increased concentration causes reaction rate to Because more particles are present, collisions occur (3) Surface Area A greater surface area causes reaction rate to Greater surface area exposes more particles and so more Surface area can be increased by , , (4) Catalyst A catalyst is a substance, atom or molecule, that increases the rate of a reaction by the Catalysts affect forward and reverse reactions to overcome can occur EQUILIBRIUM Reactions that occur in both directions are called reactions. At , the forward and reverse reactions occur at the same This is NOT the same thing as equal ; some amounts of reactants and products must be present, but generally not equal amounts of each. Le Chatelier’s Principle A system at equilibrium tends to remain at equilibrium When something is done to disturb this equilibrium, the system will respond in a way that will return it to its equilibrium state 1) Change Concentration When something is added, shift away from that thing When something is removed, shift towards that thing Only amounts of (aq) and (g) count; (s) and (l) have NO EFFECT Adding a substance not in the reaction will have NO EFFECT Example: CO2(g) + H2(g) ↔ CO(g) + H2O(l) a) Add more carbon dioxide b) Remove hydrogen gas as it forms c) Add more water 2) Change Volume (of container); Change Pressure If volume is reduced (or pressure is increased), shift to side with less gas particles If volume is increased (or pressure is decreased), shift to side with more gas particles Example: CO2(g) + H2(g) ↔ CO(g) + H2O(l) a) volume is reduced b) pressure is reduced c) volume is increased d) pressure is reduced 3) Change Temperature If temperature is increased, shift to side without heat If temperature is decreased, shift to side with heat Example: CO2(g) + H2(g) + heat ↔ CO(g) + H2O(l) a) Temperature is increased b) Temperature is decreased **Any other change will have NO EFFECT on the position of equilibrium** Equilibrium Constants K = [Products] [Reactants] Example: aA + bB ↔ cC + dD **Solids and liquids do not appear in equilibrium expressions!! Examples a) Dinitrogen tetroxide, a colorless gas, and nitrogen dioxide, a dark brown gas, exist in equilibrium with each other: N2O4(g) ↔ 2 NO2(g) A liter of a gas mixture at 10°C at equilibrium contains 0.0045 mol of dinitrogen tetroxide and 0.030 mol of nitrogen dioxide. Write the expression for the equilibrium constant and calculate Keq for this reaction. b) Analysis of an equilibrium mixture of nitrogen, hydrogen, and ammonia contained in a 1-liter flask at 300C gives the following results: hydrogen 0.15 mol, nitrogen 0.25 mol, and ammonia 0.10 mol. Calculate Keq for this reaction. N2(g) + 3 H2(g) ↔ 2 NH3(g) K gives information about the extent of the reaction: K > 1 means are favored at equilibrium K < 1 means are favored at equilibrium